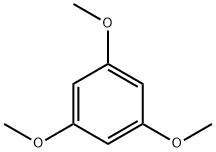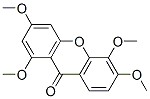
1,3,5,6-Tetramethoxy-9H-xanthen-9-one synthesis
- Product Name:1,3,5,6-Tetramethoxy-9H-xanthen-9-one
- CAS Number:3660-86-4
- Molecular formula:
- Molecular Weight:0
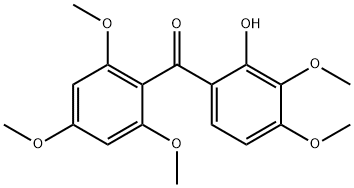
42833-67-0
0 suppliers
inquiry

3660-86-4
0 suppliers
inquiry
Yield:3660-86-4 2.24 g
Reaction Conditions:
with pyridine;tetramethylammonium hydroxide in water; for 36 h;Reflux;
Steps:
1 Synthesis of compound 4
5.1.2.1 1,3,5,6-Tetramethoxy-9H-xanthen-9-one (4, C17H16O6). 2,3,4-Trimethoxybenzoic acid (2.78 g, 13.14 mmol) in anhydrous benzene (60 mL) was treated with 5.0 mL of thionyl chloride and thoroughly stirred at room temperature. After 2 h, the solvent and the excess reagent were removed under reduced pressure. The residue, 2,3,4-trimethoxybenzoyl chloride was dissolved in anhydrous ether (80 mL), 1,3,5-trimethoxybenzene (2.20 g, 13.03 mmol) and AlCl3 (5.0 g) were added. After stirring for 8 h at room temperature, the mixture was hydrolyzed with ice-cold water (500 mL) containing concentrated HCl (45 mL) and extracted with CH2Cl2. Solvent removal and purification with gel-column chromatography (CHCl3) gave yellow solid 2-hydroxy-2',3,4,4',6'-pentamethoxybenzophenone (2.97 g, 8.53 mmol, 65%). The yellow solid (2.97 g, 8.53 mmol) was treated with pyridine (100 mL), H2O (50 mL) and aqueous 10% tetramethylammonium hydroxide (45 mL). The mixture was refluxed for 36 h, poured into ice, acidified with HCl, and extracted with ether. Purification with gel-chromatography (CHCl3) and recrystallization (CHCl3) gave colorless needle crystals, 1,3,5,6-tetramethoxy xanthone (2.24 g, 7.08 mmol, 83%). 1H NMR (DMSO-d6, 300 MHz): δ (ppm), 7.78 (d, J = 9.0 Hz, 1H), 7.15 (d, J = 9.1 Hz, 1H), 6.71 (d, J = 2.4 Hz, 1H), 6.49 (d, J = 2.4 Hz, 1H), 3.92 (s, 3H), 3.91 (s, 3H), 3.89 (s, 3H), 3.85 (s, 3H); 13C NMR (DMSO-d6, 150 MHz): δ (ppm), 174.9, 166.5, 163.3, 161.0, 158.3, 150.4, 137.3, 123.0, 119.0, 111.0, 107.7, 97.4, 95.2, 62.8, 58.2, 58.0, 57.9; HR-ESI-MS calcd for C17H16O6 [M + H]+ 316.0947, found 316.0949.
References:
Dai, Ming;Yuan, Xing;Kang, Jian;Zhu, Zhi-Jun;Yue, Rong-Cai;Yuan, Hu;Chen, Bing-Yang;Zhang, Wei-Dong;Liu, Run-Hui;Sun, Qing-Yan [European Journal of Medicinal Chemistry,2013,vol. 69,p. 159 - 166]
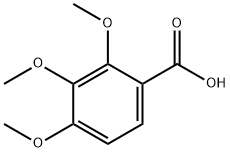
573-11-5
242 suppliers
$6.00/5g

3660-86-4
0 suppliers
inquiry

7169-07-5
13 suppliers
inquiry

3660-86-4
0 suppliers
inquiry