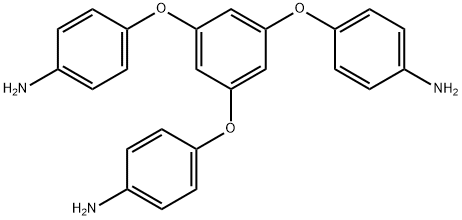
1,3,5-TRIS(4-AMINOPHENOXY)BENZENE (135TAPOB) synthesis
- Product Name:1,3,5-TRIS(4-AMINOPHENOXY)BENZENE (135TAPOB)
- CAS Number:102852-92-6
- Molecular formula:C24H21N3O3
- Molecular Weight:399.44
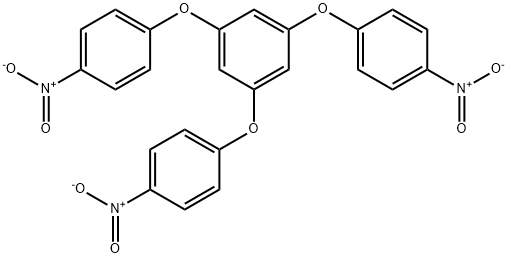
102852-91-5
16 suppliers
inquiry

102852-92-6
99 suppliers
$11.00/100mg
Yield:102852-92-6 95%
Reaction Conditions:
with hydrogen;palladium on carbon in ethyl acetate at 20; for 16 h;
Steps:
1.2
7.0 g (14.3 mmol) of the nitro-form A and 700 mg of Pd/C as a hydrogenation catalyst were suspended in EtOAc (140 mL) serving as a second dispersion medium to prepare a second dispersion. The second dispersion was stirred under a hydrogen atmosphere at room temperature for 16 hours. Following the stirring, Pd/C was removed by filtering the second dispersion, and the filtrate was concentrated using a rotary evaporator to yield a crude amine-form A. The obtained crude amine-form was purified by silica gel column chromatography (EtOAc) to yield an amine-form A (5.43 g) in the form of a brown solid (second step). The analysis results for the obtained amine-form A by NMR were as follows. 1H-NMR (600 MHz, CDCl3, 25°C): δ 6.15 (s, 3H), 6.64 (t, J = 8.2 Hz, 6H), 6.84(d, J = 8.2 Hz, 6H) 13C-NMR (600 MHz, CDCl3, 25°C): δ 100.3, 116.5, 121.5, 143.2, 148.1, 161.1 From the above results, it was confirmed that the amine-form A was 1,3,5-tris(p-aminophenoxy)benzene represented by the aforementioned general formula (4). Further, the yield of the amine-form A was 95%. The yield is a percentage derived by dividing the number of moles for the obtained amine-form A with the number of moles for the nitro-form A.
References:
EP2332906,2011,A1 Location in patent:Page/Page column 10; 11
108-70-3
253 suppliers
$21.00/100g
123-30-8
620 suppliers
$10.00/5g

102852-92-6
99 suppliers
$11.00/100mg
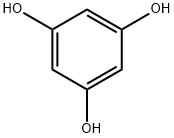
108-73-6
530 suppliers
$13.00/5g

102852-92-6
99 suppliers
$11.00/100mg
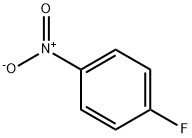
350-46-9
522 suppliers
$10.60/1gm:

102852-92-6
99 suppliers
$11.00/100mg

108-73-6
530 suppliers
$13.00/5g
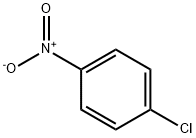
100-00-5
46 suppliers
$14.00/25g

102852-92-6
99 suppliers
$11.00/100mg