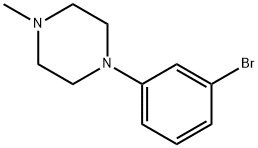
1-(3-broMophenyl)-4-Methylpiperazine synthesis
- Product Name:1-(3-broMophenyl)-4-Methylpiperazine
- CAS Number:747413-17-8
- Molecular formula:C11H15BrN2
- Molecular Weight:255.15
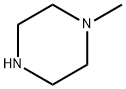
109-01-3
672 suppliers
$5.00/5G
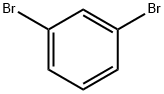
108-36-1
454 suppliers
$10.00/1g

747413-17-8
30 suppliers
$171.19/100mg
Yield:747413-17-8 78%
Reaction Conditions:
with 2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl;1,8-diazabicyclo[5.4.0]undec-7-ene;sodium t-butanolate;bis(dibenzylideneacetone)-palladium(0) in toluene at 60 - 100;
Steps:
31.2
1- (3-BROMO-PHENYL)-4-METHYL-PIPERAZINE 1,3-Dibromobenzene (0. 90MOL, 7.49 MMOL), N-methylpiperazine (0. 28ML, 2. 50MMOL) and anhydrous toluene (7ml) were added by syringe to a dry, argon filled flask. The solution was thoroughly mixed before BINAP (47mg) and Pd2dba3 (23mg) were delivered and the flask refilled with Argon and DBU (0.93g, 2.5 equiv. ) added via syringe. The reaction mixture was warmed to 60 C before freshly ground sodium tertbutoxide was added in one portion to start the reaction. The reaction was left stirring at 60 C overnight and the TLC analysis appeared to show that some piperazine was still present so the reaction was heated to 100 C and stirred for another 24hrs after which it was partitioned between EtOAc (20ML) and water (20ML). The aqueous layer was extracted again with EtOAc and the combined organics were washed with 1.6M HCI solution (2 x 10MOL). The acidic solution containing the product was then basified first with a similar volume of 1 M NAOH solution to acid solution and then carefully solid sodium bicarbonate was added to make the pH=8.5 before extraction back into EtOAc (2 X 15ML), which was washed with brine, dried over MGS04 and evaporated to dryness to provide 0.50g (78% yield) of the pure product as a yellow oil. LCMS TR = 4.55, MS m/z 255. 4/257. 3 [M+H] +
References:
WO2004/72051,2004,A1 Location in patent:Page 55

109-01-3
672 suppliers
$5.00/5G
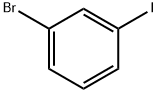
591-18-4
396 suppliers
$5.00/5g

747413-17-8
30 suppliers
$171.19/100mg

50-00-0
896 suppliers
$10.00/25g
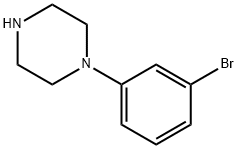
31197-30-5
126 suppliers
$20.00/250mg

747413-17-8
30 suppliers
$171.19/100mg
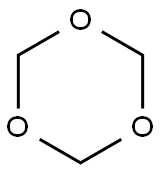
110-88-3
263 suppliers
$13.00/100g

31197-30-5
126 suppliers
$20.00/250mg

747413-17-8
30 suppliers
$171.19/100mg