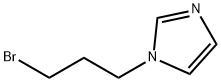
1-(3-bromopropyl)-1H-imidazole(SALTDATA: HBr) synthesis
- Product Name:1-(3-bromopropyl)-1H-imidazole(SALTDATA: HBr)
- CAS Number:144385-79-5
- Molecular formula:C6H9BrN2
- Molecular Weight:189.05

288-32-4
1053 suppliers
$5.00/25g

109-64-8
515 suppliers
$10.00/5g

144385-79-5
14 suppliers
$65.00/100mg
Yield:144385-79-5 78%
Reaction Conditions:
Stage #1: 1H-imidazolewith potassium carbonate in N,N-dimethyl-formamide at 20; for 0.333333 h;
Stage #2: 1,3-dibromo-propane in N,N-dimethyl-formamide at 20; for 30 h;Reagent/catalyst;
Steps:
3.2 General procedure for N-alkylationof heterocycles with 1,3-dibromopropane
General procedure: The general procedure for the Nalkylation of heterocycles was as follows.22 Heterocycle (1 mmol) wasdissolved in 5 ml anhydrous DMF. Then complex(0.05 mmol) and K2CO3 was added to the solution atroom temperature. Shortly afterwards (20 min),1,3-dibromopropane (1 mmol) was added in portions to the reaction mixture. The reaction was stirred at room temperature. The inorganic salt was removed byfiltration and rinsed twice with dichloromethane. The solution was poured into water and extracted withdichloromethane (2 x 25 ml). The combined organiclayers were washed with brine, dried over anhydroussodium sulphate, filtered, and concentrated in vacuoresulting in the formation of the product in 92 % yieldwith coupling ratio 1:1 and product yield 78 % withcoupling ratio 2:1. Nalkylation of heterocycles with1,3-dibromopropane having 1:1 coupling proportion(Scheme-2) is easier than 2:1 coupling proportion(Scheme-3).
References:
Chavan, Sanjay;Gaikwad, Gautam;Hegade, Sujit;Jadhav, Yuvraj;Mulik, Ganpatrao [Journal of Chemical Sciences,2020,vol. 132,# 1]
51390-23-9
45 suppliers
$45.00/25mg

144385-79-5
14 suppliers
$65.00/100mg
18999-46-7
13 suppliers
inquiry

144385-79-5
14 suppliers
$65.00/100mg