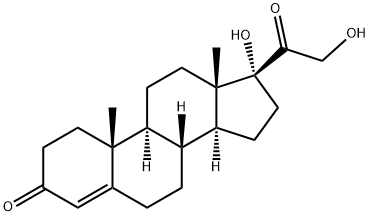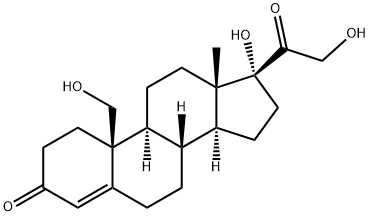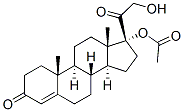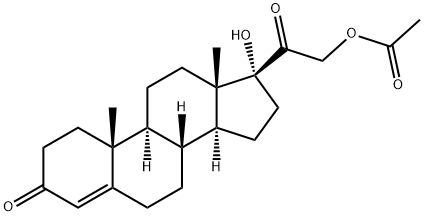
Hydrocortisone synthesis
- Product Name:Hydrocortisone
- CAS Number:50-23-7
- Molecular formula:C21H30O5
- Molecular Weight:362.47
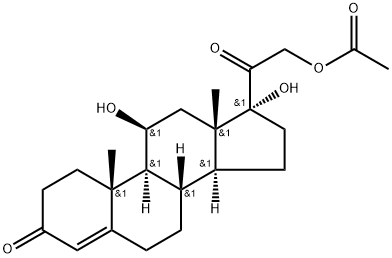
50-03-3
604 suppliers
$32.70/1g

50-23-7
786 suppliers
$24.00/1g
Yield:50-23-7 94.82%
Reaction Conditions:
with sodium methylate in methanol at 20; for 0.5 h;
Steps:
2 Synthesis of 11, 17, 21-trihydroxy-pregn-4-ene-3, 20-dione
Compound 2 was synthesized by dissolving 29 mg (0.071 mmol)of compound (1) in 1.5 mL of methanol followed by addition of0.3 mL of sodium methoxide as a catalyst. The mixture was kept atroom temperature for 30 min. The reaction mixture was neutralizedwith IR 120H resin and filtered. Filtrate was concentratedand compound 2 was purified with column chromatography usingChloroform/methanol as eluent yielding 27.5 mg (94.82%) of compound2 in pure state. m.p: 490e491 K, Molecular formula:C21H30O5, 1H NMR in CDCl3 at 300 MHz: d 7.260(D, s, CDCl3), d 0.962(1H, s, H-18), d 1.054, 1.017 (1H, dd, H-9, J 3.3 Hz), d 1.442 (1H, s,H-19), d 3.061 (1H, broad signal, OH-21), d 4.337(1H, d, J 19.8 Hz,H-21A), d 4.686 (1H, d, J 19.8 Hz, H-21B) d 4.468e4.499 (1H,q,J 3 Hz, H-11), d 5.689 (1H, d, H-4, J 1.2 Hz). FT-IR nmax (in cm1):3381.44, 2950.5, 2924.74, 2859.79, 1711.34, 1658.76, 1462.88,1378.35, 1271.13, 1235.05, 1090.72, 1025.77, 869.07, 763.91, 743.29.ESI-MS: 385, 362, 346, 302.
References:
Sethi, Arun;Singh, Ranvijay Pratap;Prakash, Rohit;Amandeep [Journal of Molecular Structure,2017,vol. 1130,p. 860 - 866]
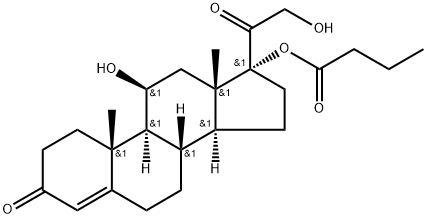
13609-67-1
254 suppliers
$36.00/200mg

50-23-7
786 suppliers
$24.00/1g