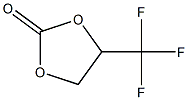
1,3-Dioxolan-2-one, 4-(trifluoromethyl)-, (+)- synthesis
- Product Name:1,3-Dioxolan-2-one, 4-(trifluoromethyl)-, (+)-
- CAS Number:167951-81-7
- Molecular formula:C4H3F3O3
- Molecular Weight:156.06
Yield:167951-81-7 Ca. 175 g
Reaction Conditions:
with 1H-imidazole in tert-butyl methyl ether at 50;Inert atmosphere;
Steps:
2 EXAMPLE 2
A glass reactor equipped with drain valve, internal temperature probe, addition port, condenser, and gas inlet/outlet adapters was flushed with nitrogen.
The jacket of the reactor was connected to a heating/chilling circulator.
The gas outlet port was connected to a silicon oil bubbler.
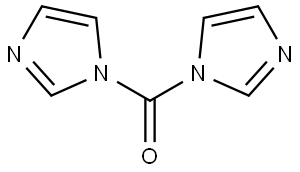
The reactor was charged with CDI and MTBE and heated to about 50° C.
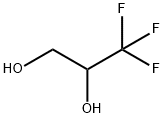
A solution of trifluoropropylene glycol was added at 2-3 ml/min.
The reactor was maintained at about 50° C. overnight, then cooled to about 5° C. over about 3 hours.
At 36° C., the solution was seeded by withdrawing 1 ml and rapidly cooling to form solid imidazole.
This cooled suspension was returned to the reactor.
Imidazole immediately began to crystallize in the reactor.
Once cooled completely, the supernatant liquid was removed.
The solids were washed with 1:1 MTBE:heptane.
These solutions were then separately roto-vaped to a thin oil. GC analysis concluded they were the same composition (wrt imidazole concentration) and they were then combined.The next day, this was distilled. Given a starting substrate of about 200 grams of the trifluoropropylene glycol, and about 260 grams CDI, product yield was about 175 g
References:
US2018/79708,2018,A1 Location in patent:Paragraph 0045; 0046
431-39-0
37 suppliers
$29.00/100mg
32315-10-9
438 suppliers
$10.00/1g

167951-81-7
2 suppliers
inquiry
359-41-1
175 suppliers
$7.00/1g

124-38-9
132 suppliers
$214.00/14L

167951-81-7
2 suppliers
inquiry

431-39-0
37 suppliers
$29.00/100mg