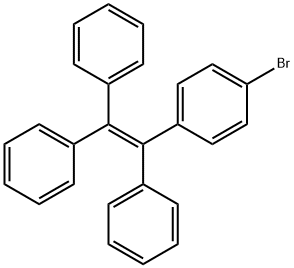
1-(4-BroMophenyl)-1,2,2-triphenylethylene synthesis
- Product Name:1-(4-BroMophenyl)-1,2,2-triphenylethylene
- CAS Number:34699-28-0
- Molecular formula:C26H19Br
- Molecular Weight:411.33
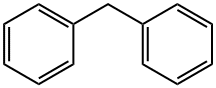
101-81-5
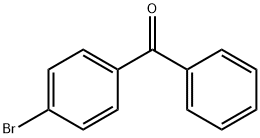
90-90-4

34699-28-0
General procedure for the synthesis of 1-(4-bromophenyl)-1,2,2-tristyrylene from diphenylmethane and 4-bromobenzoylbenzene: 16.7 mL (0.1 mol) of diphenylmethane was dissolved in 300 mL of tetrahydrofuran (THF) under argon protection and added to 500 mL of a three-neck flask. The flask was magnetically stirred under ice-salt bath conditions for 40 min, and 54.5 mL (0.12 mol) of n-butyllithium solution was slowly added dropwise, and the ice-salt bath stirring was continued for 40 min. Subsequently, 13.0555 g (0.05 mol) of 4-bromobenzophenone (bis(4-bromophenyl)methanone) was added and the reaction mixture was stirred for 12 hours at room temperature. Upon completion of the reaction, the reaction was quenched by the addition of 250 mL of saturated ammonium chloride (NH4Cl) solution and stirred for 10 minutes. The reaction solution was transferred to a separatory funnel, and the organic phase was separated and dried with 300 g of anhydrous sodium sulfate. The dried organic phase was concentrated to dryness in a rotary evaporator. The resulting solid was dissolved in an appropriate amount of solvent, 5 g of p-toluenesulfonic acid was added, and the reaction was heated to 110 °C and refluxed for 12 hours. At the end of the reaction, the reaction solution was cooled to room temperature, dried and concentrated. The dried powdery product was obtained by rotary evaporation and further dried under vacuum at 60 °C for 24 h to give the white intermediate Si-Br in 89% yield.

101-81-5
420 suppliers
$7.00/25g

90-90-4
239 suppliers
$10.00/5g

34699-28-0
99 suppliers
$8.00/100mg
Yield:34699-28-0 89%
Reaction Conditions:
Stage #1:Diphenylmethane;(4-bromophenyl)(phenyl)methanone with n-butyllithium in tetrahydrofuran for 1.33333 h;Inert atmosphere;Cooling with ice;
Stage #2:(4-bromophenyl)(phenyl)methanone in tetrahydrofuran at 20; for 12 h;
Steps:
3.1 (1) Synthesis of intermediate 1-bromo-4- (1,2,2-triphenylvinyl) benzene. (Si-Br)
16.7 mL (0.1 mol) of diphenylmethane and 300 ml of tetrahydrofuran (THF) were added to a solution of500ml three-necked flask, magnetic stirring and argon, ice salt bath 40min, to the three bottles by adding 54.5mL (0.12mol) n-butyl lithium solution, continue ice salt bath 40min, add 13.0555g4-bromobenzophenone(Bis (4-bromophenyl) methanone, 0.05),After the reaction was carried out at room temperature for 12 h, 250 ml of saturated ammonium chloride (NH4Cl) solution was added to the three-necked flask. After stirring for 10 min, the reaction solution was poured into a separatory funnel, the supernatant was taken and the water in the solution was adsorbed with 300 g of anhydrous sodium sulfate. And then heated to 110 ° C. After adding 5 g of p-toluenesulfonic acid, the mixture was refluxed for 12 hours. After the reaction was stopped, the reaction solution was dried and dried. The reaction mixture was heated to 110 ° C, The product was spin dried to dry the powder and the powder was dried in vacuo at 60 ° C for 24 h to give the white intermediate Si-Br in 89% yield.
References:
Sun Yat-sen University;Zhang, Yi;Liu, Yiwu;Xu, Jiarui;Wu, Xinhui;Shi, Jie;Liu, Siwei;Chi, Zhenguo CN104341311, 2016, B Location in patent:Paragraph 0057; 0058; 0059
119-61-9
821 suppliers
$5.00/10g

90-90-4
239 suppliers
$10.00/5g

34699-28-0
99 suppliers
$8.00/100mg

101-81-5
420 suppliers
$7.00/25g

34699-28-0
99 suppliers
$8.00/100mg

90-90-4
239 suppliers
$10.00/5g

34699-28-0
99 suppliers
$8.00/100mg
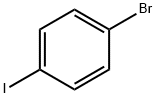
589-87-7
540 suppliers
$10.00/1g
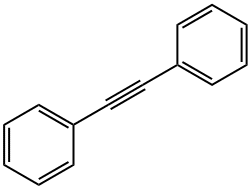
501-65-5
220 suppliers
$16.00/5g
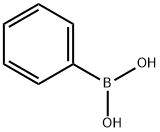
98-80-6
745 suppliers
$5.00/5g

34699-28-0
99 suppliers
$8.00/100mg