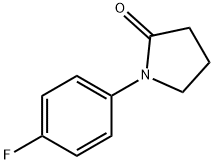
1-(4-FLUOROPHENYL)-2-PYRROLIDINONE synthesis
- Product Name:1-(4-FLUOROPHENYL)-2-PYRROLIDINONE
- CAS Number:54660-08-1
- Molecular formula:C10H10FNO
- Molecular Weight:179.19
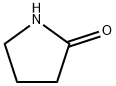
616-45-5
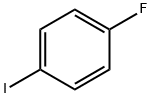
352-34-1

54660-08-1
General procedure for the synthesis of 1-(4-fluorophenyl)-2-pyrrolidinone from 2-pyrrolidinone and p-fluoroiodobenzene: N-nucleophilic reagent (2.21 mmol), Cu2O (Sigma-Aldrich, 99.99% purity, 0.147-0.294 mmol), K3PO4 (2.94 mmol), aryl halide (1.47 mmol), phase transfer catalyst (0.147-0.294 mmol) and water (0.40 mL) were sequentially added to the reaction vial and sealed by installing a screw cap. The reaction mixture was stirred in a closed system at 130 °C for 24 h under air atmosphere. Upon completion of the reaction, the non-homogeneous mixture was cooled to room temperature and diluted with dichloromethane. The resulting solution was filtered directly through diatomaceous earth. The organic extracts were combined, dried with anhydrous Na2SO4 and concentrated under reduced pressure to remove the solvent. The crude product was purified by silica gel column chromatography to afford the N-arylated product 1-(4-fluorophenyl)-2-pyrrolidinone. The structure and purity of the product were confirmed by 1H NMR and 13C NMR spectral analysis.

616-45-5
405 suppliers
$14.00/25g
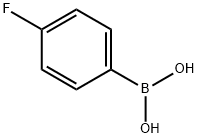
1765-93-1
552 suppliers
$9.00/1g

54660-08-1
54 suppliers
$20.00/250mg
Yield:54660-08-1 97%
Reaction Conditions:
Stage #1: 2-pyrrolidinon;4-fluoroboronic acidwith copper(l) iodide in dimethyl sulfoxide at 20; for 0.166667 h;
Stage #2: with tert.-butylhydroperoxide in water;dimethyl sulfoxide at 60; for 1 h;
Steps:
Typical Procedure
General procedure: Aryl boronic acid (1.0 mmol), CuI (5 mol%),amide (3.0 mmol), and DMSO (1.0 mL) were added to a reactionvial, and the mixture was stirred at room temperature for10 min. A 70% aqueous solution of TBHP (1.1 mmol) was addedto the reaction mixture dropwise over 5 min. The reaction vialwas then immersed in a preheated oil bath and the progress ofreaction was followed by TLC. Upon completion of reaction, thecooled mixture was partitioned between water and ethyl acetate.The aqueous layer was further extracted with ethyl acetate,and the combined organic layers were washed with brine,dried over Na2SO4, filtered, and concentrated in vacuo. Theresidue was purified by column chromatography on silica gel(hexane-ethyl acetate) to give the desired N-aryl lactam
References:
Bathini, Thulasiram;Rawat, Vikas Singh;Sreedhar, Bojja [Synlett,2015,vol. 26,# 10,art. no. ST-2015-D0213-L,p. 1348 - 1351] Location in patent:supporting information

4280-36-8
0 suppliers
inquiry

54660-08-1
54 suppliers
$20.00/250mg

616-45-5
405 suppliers
$14.00/25g

352-34-1
376 suppliers
$7.00/5g

54660-08-1
54 suppliers
$20.00/250mg

616-45-5
405 suppliers
$14.00/25g
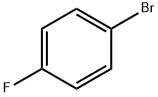
460-00-4
635 suppliers
$6.00/10g

54660-08-1
54 suppliers
$20.00/250mg

75-09-2
1253 suppliers
$10.00/25ML

60252-77-9
10 suppliers
inquiry

54660-08-1
54 suppliers
$20.00/250mg