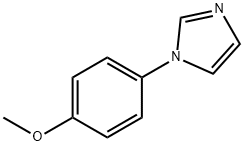
1-(4-METHOXYPHENYL)-1H-IMIDAZOLE synthesis
- Product Name:1-(4-METHOXYPHENYL)-1H-IMIDAZOLE
- CAS Number:10040-95-6
- Molecular formula:C10H10N2O
- Molecular Weight:174.2

288-32-4
1039 suppliers
$5.00/25g
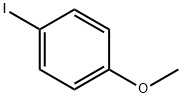
696-62-8
358 suppliers
$5.00/1g

10040-95-6
111 suppliers
$30.00/100mg
100-66-3
539 suppliers
$10.00/5G
Yield:-
Reaction Conditions:
with [Cu2(1,4-benzenedicarboxylate)2(1,4-diazabicyclo[2.2.2]octane)]n;potassium tert-butylate in N,N-dimethyl-formamide at 120; for 3 h;Green chemistry;Ullmann Condensation;
Steps:
2.3 Catalytic Studies
General procedure: The Cu2(BDC)2(DABCO) was used as a catalyst for the N-arylation of 4'-iodoacetophenone and imidazole. A mixture of 4'-iodoacetophenone (0.246 g, 1 mmol) and n-hexadecane (0.1 ml, 0.88 mmol) as an internal standard in DMF (4 ml) was added into a 25 mL flask containing the Cu2(BDC)2(DABCO) catalyst and KOtBu (0.224 g, 2 mmol) as a base. The catalyst concentration was calculated with respect to the copper/4'-iodoacetophenone molar ratio. Imidazole (0.204 g, 3 mmol) was then added, and the resulting mixture was stirred at 120 °C for 3 h. Reaction conversion was monitored by withdrawing aliquots from the reaction mixture at different time intervals, quenching with aqueous NaOH solution (1 % w/w, 1 ml). The organic components were then extracted into diethyl ether (2 ml), dried over anhydrous Na2SO4, analyzed by GC with reference to n-hexadecane. The product identity was further confirmed by GC-MS. To investigate the recyclability of Cu2(BDC)2(DABCO), the catalyst was separated from the reaction mixture by simple centrifugation, washed with copious amounts of DMF, dried under air at room temperature for 1 h, and reused if necessary. For the leaching test, a catalytic reaction was stopped after 30 min, analyzed by GC, and centrifuged to remove the solid catalyst. The reaction solution was then stirred for a further 150 min. Reaction progress, if any, was monitored by GC as previously described. For homogeneity test, Cu(NO3)2*3H2O (5 mol %), H2BDC (5 mol %), and DABCO (5 mol %) were used as catalytic mixture for the reaction. After 30 min of reaction time, the reaction mixture was centrifuged and the mother liquor was further stirred for a further 150 min under control of GC. Furthermore, copper content of the mother liquor was confirmed by ICP analysis.
References:
Nguyen, Tung T.;Phan, Nam T. S. [Catalysis Letters,2014,vol. 144,# 11,p. 1877 - 1883]

288-32-4
1039 suppliers
$5.00/25g
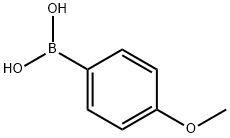
5720-07-0
468 suppliers
$6.00/5g

10040-95-6
111 suppliers
$30.00/100mg

288-32-4
1039 suppliers
$5.00/25g
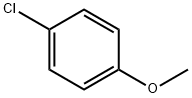
623-12-1
294 suppliers
$10.00/25g

10040-95-6
111 suppliers
$30.00/100mg

288-32-4
1039 suppliers
$5.00/25g

696-62-8
358 suppliers
$5.00/1g

10040-95-6
111 suppliers
$30.00/100mg

288-32-4
1039 suppliers
$5.00/25g
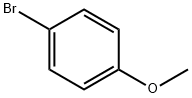
104-92-7
433 suppliers
$10.00/10g

10040-95-6
111 suppliers
$30.00/100mg