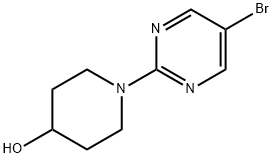
1-(5-BROMOPYRIMIDIN-2-YL)-4-PIPERIDINOL synthesis
- Product Name:1-(5-BROMOPYRIMIDIN-2-YL)-4-PIPERIDINOL
- CAS Number:887425-47-0
- Molecular formula:C9H12BrN3O
- Molecular Weight:258.12
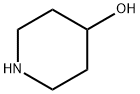
5382-16-1
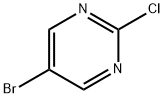
32779-36-5

887425-47-0
Step 1: 4-hydroxypiperidine (1.93 g, 10 mmol), diisopropylethylamine (5.22 mL, 30 mmol), 5-bromo-2-chloropyrimidine (1.01 g, 10 mmol), and acetonitrile (50 mL) were added to a round-bottomed flask under nitrogen protection. The reaction mixture was heated to reflux for 15 hours and then concentrated under reduced pressure to remove the solvent. The crude product was dissolved in dichloromethane (150 mL), washed sequentially with water (2 x 20 mL), saturated saline (1 x 20 mL), dried over anhydrous sodium sulfate and filtered. The filtrate was concentrated under reduced pressure and purified by silica gel column chromatography to afford 1-(5-bromo-2-pyrimidinyl)-4-hydroxypiperidine (16) (2.55 g, 99% yield) as a white solid.1H NMR (400 MHz, DMSO-d6) δ: 8.34 (s, 2H), 4.73 (d, J = 4.0 Hz, 1H), 4.17-4.12 (m 2H), 3.74-3.68 (m, 1H), 3.28-3.22 (m, 2H), 1.76-1.70 (m, 2H), 1.33-1.25 (m, 2H); LCMS (ESI): m/z 260 [M+H]+.

5382-16-1
0 suppliers
$9.00/5G

32779-36-5
631 suppliers
$5.00/5g

887425-47-0
49 suppliers
$60.00/50mg
Yield:887425-47-0 99%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in acetonitrile; for 15 h;Heating / reflux;
Steps:
5.1
Step 1 : 1-(5-Bromo-2-pyrimidinyl)-4-piperidinol (16)A round-bottomed flask was charged, under N2, with 4-hydroxypiperidine (1.93 g, 10 mmol), diisopropylethylamine (5.22 ml_, 30 mmol), 5-bromo-2-chloropyrimidine (1.01 g, 10 mmol), and acetonitrile (50 ml_). The mixture was refluxed for 15 h and then concentrated under reduced pressure. The crude product was redissolved in CH2CI2 (150 ml), washed with H2O (2 x 20 ml_), brine (1 x 20 ml_), dried over Na2SO4, and filtered. The filtrate was concentrated under reduced pressure and the crude material was purified by flash SiO2 column chromatography to afford 2.55 g (99%) of the title compound 16 as a white solid. 1H NMR (400 MHz, DMSO-d6): δ 8.34 (s, 2 H), 4.73 (d, J = 4.0 Hz, 1 H), 4.17 - 4.12 (m, 2 H), 3.74 - 3.68 (m, 1 H), 3.28 - 3.22 (m, 2 H), 1.76 - 1.70 (m, 2 H), 1.33 - 1.25 (m, 2 H); LCMS (ESI): m/z 260 (M + H)+.
References:
WO2008/8895,2008,A1 Location in patent:Page/Page column 70