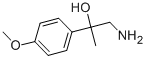
1-amino-2-(4-methoxyphenyl)propan-2-ol synthesis
- Product Name:1-amino-2-(4-methoxyphenyl)propan-2-ol
- CAS Number:305448-36-6
- Molecular formula:C10H15NO2
- Molecular Weight:181.23
Yield:305448-36-6 24%
Reaction Conditions:
with hydrogenchloride;zinc diiodide in tetrahydrofuran;methanol;sodium hydroxide;
Steps:
34 Preparation of 1-amino-2-(4-methoxyphenyl)propan-2-ol
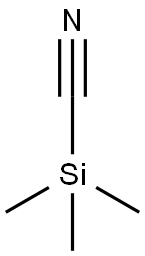
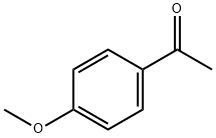
Preparation of 1-amino-2-(4-methoxyphenyl)propan-2-ol Into a 100 mL single-neck flask, 28.5 mL of trimethylsilyl cyanide was added dropwise to 10 g of 4'-methoxyacetophenone and 2.28 g of zinc iodide, while stirring under a nitrogen atmosphere at room temperature. The reaction was allowed to stir at this temperature overnight. In the morning, the mixture was diluted with methylene chloride (100 mL) and the organic layer was backwashed once with sat. Na2HCO3, dried over MgSO4, and concentrated under reduced vacuum to yield 13 g. as a tan oil. Because of possible stability problems, this material was used immediately and placed into 200 mL THF in a 500 mL single-neck flask. While stirring at room temperature under a nitrogen atmosphere, 70 mL of BH3-THF complex was added syringe wise and the reaction was stirred overnight. In the morning, 10 mL of concentrated hydrochloric acid was added dropwise at room temperature and severe foaming was present. The mixture was then concentrated under reduced vacuum. The resulting HCl salt was liberated while being stirred in 1N NaOH and the free amine was extracted into ethyl acetate. This organic layer was washed with H2O, dried over Na2SO4, and concentrated under reduced vacuum to yield 7.31 g. of a 2 spot material. This material was purified via silica gel chromatography employing the Water's prep. 2000 and eluding with a solvent of methylene chloride/methanol 9:1 to yield intermediate title compound (3.01 g, 24%) as an oil as the top spot. (FD) M.S. 180.3 (M*-1).
References:
US2004/63680,2004,A1

1172878-66-8
16 suppliers
inquiry

305448-36-6
9 suppliers
inquiry