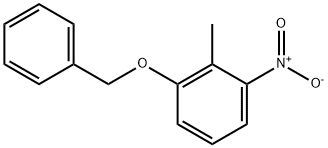
1-(Benzyloxy)-2-methyl-3-nitrobenzene synthesis
- Product Name:1-(Benzyloxy)-2-methyl-3-nitrobenzene
- CAS Number:20876-37-3
- Molecular formula:C14H13NO3
- Molecular Weight:243.26
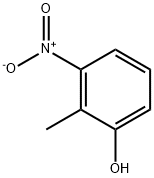
5460-31-1

100-39-0

20876-37-3
General procedure: 41.5 g (300 mmol) of potassium carbonate and 200 mL of N,N-dimethylformamide were added to a reaction vial containing 30.6 g (200 mmol) of 2-methyl-3-nitrophenol. Under argon protection, 23.8 mL (200 mmol) of benzyl bromide was slowly added dropwise to the reaction system and the reaction was stirred at room temperature for 3 hours. Upon completion of the reaction, the reaction solution was poured into 1000 mL of water, extracted with 800 mL of toluene and the extraction was repeated once (500 mL of toluene). The organic phases were combined, washed sequentially with water and saturated aqueous sodium chloride, and dried over anhydrous magnesium sulfate. The organic phase was concentrated under reduced pressure to give 49.3 g of yellow powdered 1-benzyloxy-2-methyl-3-nitrobenzene in quantitative yield. rf value: 0.48 (unfolding reagent: n-hexane/ethyl acetate=4:1, v/v). Mass spectrum (CI, m/z): 244 [M+1]+. 1H-NMR (CDCl3, δ ppm): 2.42 (s, 3H), 5.13 (s, 2H), 7.08-7.11 (m, 1H), 7.21-7.27 (m, 1H), 7.32-7.44 (m, 6H).

100-39-0
431 suppliers
$10.00/10 g

20876-37-3
107 suppliers
$9.00/250mg
Yield: 100%
Reaction Conditions:
Stage #1:3-nitro-o-cresol with potassium carbonate in N,N-dimethyl-formamide
Stage #2:benzyl bromide in N,N-dimethyl-formamide at 20; for 3 h;
Steps:
22
(Referential Example 22) Synthesis of 2-benzyloxy-6-nitrotoluene (referential compound 22-1) Potassium carbonate (41.5 g, 300 mmol) and 200 ml of N,N-dimethylformamide were added to 30.6 g (200 mmol) of 2-methyl-3-nitrophenol. After that, 23.8 ml (200 mmol) of benzyl bromide was added thereto with stirring in an argon stream and the mixture was stirred at room temperature for 3 hours. After the reaction was finished, the reaction solution was poured into 1,000 ml of water and and the mixture was extracted with 800 ml of toluene and 500 ml of the same for two times. The organic layer was successively washed with water and a saturated aqueous solution of sodium chloride, dried over anhydrous magnesium sulfate and concentrated in vacuo to give 49.3 g of the title compound as yellow powder (yield: quantitative). Rf value: 0.48 (n-hexane: ethyl acetate = 4:1 (v/v)) Mass spectrum (CI, m/z): 244 (M+ + 1) 1H-NMR spectrum (CDCl3, δ ppm): 2.42 (s, 3H), 5.13 (s, 2H), 7.08-7.11 (m, 1H), 7.21-7.27 (m, 1H), 7.32-7.44 (m, 6H)
References:
Ube Industries, Ltd.;SANTEN PHARMACEUTICAL CO., LTD. EP1679308, 2006, A1 Location in patent:Page/Page column 42

5460-31-1
293 suppliers
$6.00/1g

100-39-0
431 suppliers
$10.00/10 g

20876-37-3
107 suppliers
$9.00/250mg

5460-31-1
293 suppliers
$6.00/1g

100-44-7
660 suppliers
$13.50/250G

20876-37-3
107 suppliers
$9.00/250mg
83-42-1
279 suppliers
$9.00/5g

20876-37-3
107 suppliers
$9.00/250mg
4837-88-1
239 suppliers
$12.72/1gm:

20876-37-3
107 suppliers
$9.00/250mg