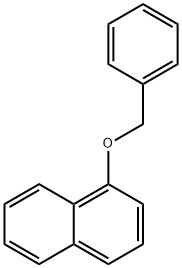
1-Benzyloxynaphthalene synthesis
- Product Name:1-Benzyloxynaphthalene
- CAS Number:607-58-9
- Molecular formula:C17H14O
- Molecular Weight:234.29
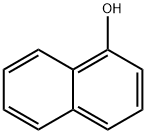
90-15-3
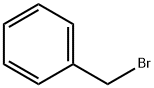
100-39-0

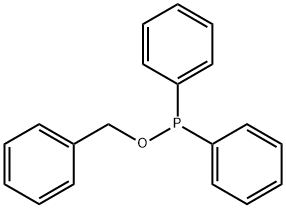
28178-96-3

607-58-9
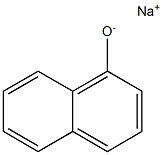
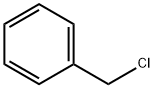
General procedure for the synthesis of compounds (CAS:28178-96-3) and 1-(benzyloxy)naphthalene from 1-naphthol and benzyl bromide: 0.14 g (1.0 mmol) of a mixture of 1-naphthol and 2-naphthol (1) and (6), mixed with 1.0 mmol of an alkali metal carbonate (0.14 g of K2CO3 or 0.33 g of Cs2CO3), was specified by 11.4 mg (0.05 mmol) of TEBAC and 1.2 mmol of alkyl halide (0.14 ml of benzyl bromide, 0.10 ml of ethyl iodide, 0.12 ml of butyl bromide, or 0.11 ml of isopropyl bromide) were added to a closed vial and reacted at 125°C for the appropriate reaction time using a 20-30 W light source or in a CEM Discover [300 W] microwave reactor. time. After completion of the reaction, the mixture was dissolved in 25 ml of ethyl acetate and the suspension was filtered. The volatile components were removed by evaporation to give the crude product. The crude product was initially purified by passing through a silica gel layer of about 2-3 cm thickness, using ethyl acetate as eluent, and the product was analyzed by GC-MS or GC. Similar reactions were carried out under 3 ml MeCN as solvent. The post-treatment step was similar to the solvent-free alkylation reaction described above, but in this case the addition of ethyl acetate was not necessary. Compounds 2, 7, 8, 10a-c and 13a, b can be obtained from the above reactions mainly in pure form by repetitive chromatography.Control experiments were carried out using benzyl bromide under conventional heating conditions with similar procedure.
Yield:607-58-9 91%
Reaction Conditions:
with potassium carbonate in acetonitrile at 125; for 0.333333 h;Microwave irradiation;Temperature;
Steps:
General Procedure for S-L Phase Alkylation of 1- and 2-Naphthol (1) and (6) under Solventless and MWConditions
General procedure: A mixture of 0.14 g (1.0 mmol) of 1- and 2-naphthol (1)and (6), in most cases 1.0 mmol of alkali carbonate (0.14 g of K2CO3 or 0.33 g of Cs2CO3), in certain cases 11.4 mg(0.05 mmol) of TEBAC and 1.2 mmol of alkyl halide (0.14ml of benzyl bromide, 0.10 ml of ethyl iodide, 0.12 mol ofbutyl bromide or 0.11 ml of i-propyl bromide) in a closedvial was irradiated (20-30 W) in a CEM Discover [300 W]MW reactor at 125 °C for the appropriate time. The reactionmixture was taken up in 25 ml of ethyl acetate and the suspensionwas filtered. Evaporation of the volatile componentsprovided the crude product that was passed through a thin(ca. 2-3 cm) layer of silica gel using ethyl acetate as theeluant to give an oil that was analysed by GC-MS or GC.Similar reactions were carried out in 3 ml of MeCN asthe solvent. The work-up was similar to that described forthe solventless alkylations above, but in this case, ethyl acetatedid not have to be added.The major components of the above reactions, such ascompounds 2, 7, 8, 10a-c and 13a,b were obtained in a pureform by repeated chromatography.Control experiments were performed with benzyl bromidein a similar way under conventional heating.
References:
Balint, Erika;Kovacs, Orsolya;Drahos, Laszlo;Keglevich, Gyoergy [Letters in Organic Chemistry,2013,vol. 10,# 5,p. 330 - 336]

90-15-3
743 suppliers
$9.00/5g

100-39-0
429 suppliers
$10.00/10g

28178-96-3
1 suppliers
inquiry

607-58-9
40 suppliers
$65.00/100mg
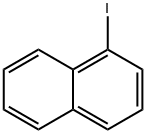
90-14-2
294 suppliers
$19.00/5g
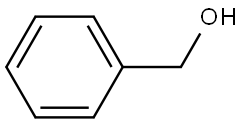
100-51-6
1432 suppliers
$5.00/100g

607-58-9
40 suppliers
$65.00/100mg