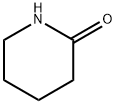
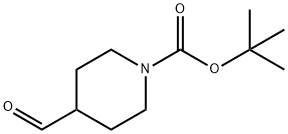
1-Boc-4-(2-oxo-piperidin-1-ylMethyl)piperidine synthesis
- Product Name:1-Boc-4-(2-oxo-piperidin-1-ylMethyl)piperidine
- CAS Number:184968-83-0
- Molecular formula:C16H28N2O3
- Molecular Weight:296.41
![1-Piperidinecarboxylic acid, 4-[[(5-chloro-1-oxopentyl)amino]methyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/184968-81-8.gif)
184968-81-8
0 suppliers
inquiry

184968-83-0
1 suppliers
inquiry
Yield:184968-83-0 96%
Reaction Conditions:
Stage #1: C16H29ClN2O3with sodium hydride in tetrahydrofuran;hexane at 0 - 23; for 22.5 h;Heating / reflux;
Stage #2: with hydrogenchloride in tetrahydrofuran;hexane;water at 0;
Steps:
2.3.2A Step 3:; Prep. 2A:
Wash NaH (3.84 g, 0.160 mol, 6.40 g of 60 wt%) with hexane (25 mL), suspend in dry THF (150 mL) and cool to 0°C under N2. Add the product (25.35 g, 0.0762 mol) of Step. 2A in dry THF (150 mL) dropwise. Stir at 23°C for 30 mins, reflux for 6 h, and stir at 23°C for 16 h. Cool to 0°C and add water (150 mL) and 1 N HCl (150 mL). Concentrate and extract with EtOAc (3x200 mL). Wash combined organic extracts with saturated aqueous NaCl, dry (MgSO4), filter and concentrate. Purify by chromatography (600 mL of flash silica gel; eluant: 5% CH3OH-CH2Cl2). Combine appropriate fractions and concentrate to give 21.62 g (0.0729 mol, 96%) of the title compound as a yellow oil. MS (FAB): m/e 297 (M+1 )
References:
EP1032561,2004,B1 Location in patent:Page 11
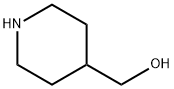
6457-49-4
355 suppliers
$18.00/25g

184968-83-0
1 suppliers
inquiry

24424-99-5
868 suppliers
$13.50/25G

184968-83-0
1 suppliers
inquiry
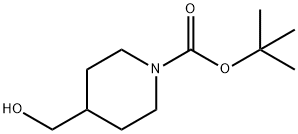
123855-51-6
407 suppliers
$6.00/5g

184968-83-0
1 suppliers
inquiry