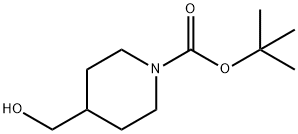
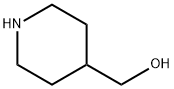
N-Boc-4-piperidinemethanol synthesis
- Product Name:N-Boc-4-piperidinemethanol
- CAS Number:123855-51-6
- Molecular formula:C11H21NO3
- Molecular Weight:215.29
N-Boc-4-piperidinemethanol is also an important intermediate of piperidine derivatives, which is mainly used in the synthesis of drugs for various neurological diseases, such as convulsions, antiepileptics, sedatives, etc. Its synthesis method is shown that:Triethylamine was added to 4-hydroxymethylpiperidine in dichloromethane in an ice bath. Di-tert butyl dicarbonate in dichloromethane was added dropwise to the reaction flask in an ice bath, and stirred at room temperature overnight. The reaction solution was quenched by adding water, washed with saturated sodium bicarbonate water and purified to obtain a white powder, N-Boc-4-piperidinemethanol.
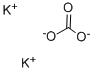
584-08-7
1252 suppliers
$5.00/100g
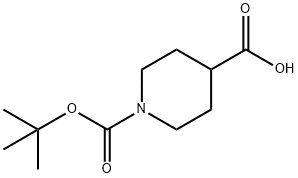
84358-13-4
379 suppliers
$5.00/5g

123855-51-6
407 suppliers
$6.00/5g
Yield:123855-51-6 84%
Reaction Conditions:
in tetrahydrofuran;water
Steps:
1.f f
f N-(tert-Butoxycarbonyl)-4-piperidinemethanol N-(tert-butoxycarbonyl)-isonipecotic acid (5.73 g, 25 mmol), as prepared in the preceding step, was dissolved in tetrahydrofuran (50 mL) and cooled to 0° C. (ice-bath). Borane-tetrahydrofuran complex (1M, 25 mL, 25 mmol) was added slowly over 30 min. The reaction mixture was stirred at 0° C. overnight and then warmed up to room temperature for 6 h. Water (10 mL) was added slowly and then K2 CO3 (5 g in 50 mL water) was added. The reaction mixture was extracted into ethyl acetate (3*50 mL). The organic phase was washed with saturated NaHCO3 (2*50 mL), brine (2*50 mL), dried over Na2 SO4, and concentrated in vacuo. The residue was then purified by flash column chromatography (1:1 hexane:ethyl acetate) to give the title compound as white crystals (4.55 g, 84%). 1H-NMR (300 MHz, CDCl3) δ 1.13 (m, 2H), 1.42 (s, 9H), 1.67 (m, 4H), 2.67 (t, 2H), 3.46 (d, 2H), 4.09 (d, 2H).
References:
3-Dimensional Pharmaceuticals, Inc. US5792769, 1998, A
142851-03-4
281 suppliers
$5.00/1g

123855-51-6
407 suppliers
$6.00/5g

84358-13-4
379 suppliers
$5.00/5g

123855-51-6
407 suppliers
$6.00/5g
6457-49-4
356 suppliers
$18.00/25g

24424-99-5
869 suppliers
$13.50/25G

123855-51-6
407 suppliers
$6.00/5g
1350060-40-0
4 suppliers
inquiry
![1-Piperidinecarboxylic acid, 4-[[(4-hydroxycyclohexyl)oxy]methyl]-, 1,1-dimethylethyl ester](/CAS/20210111/GIF/1401098-22-3.gif)
1401098-22-3
0 suppliers
inquiry

123855-51-6
407 suppliers
$6.00/5g