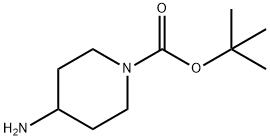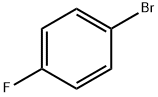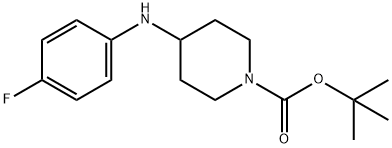
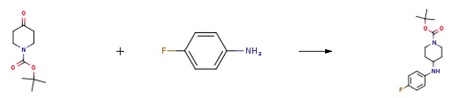
1-Boc-4-(4-fluoro-phenylamino)-piperidine synthesis
- Product Name:1-Boc-4-(4-fluoro-phenylamino)-piperidine
- CAS Number:288573-56-8
- Molecular formula:C16H23FN2O2
- Molecular Weight:294.36
Yield:288573-56-8 80%
Reaction Conditions:
with sodium cyanoborohydride;acetic acid in dichloromethane at 0 - 20; for 15 h;
Steps:
30.1
Sodium cyanoborohydride (661 mg, 10.0 mmol) was added to a methylene chloride solution (30 ml) of t-butyl 4-oxopiperidine-1-carboxylate (2.66 g, 13.1 mmol), 4-fluoroaniline (1.46 g, 13.1 mmol) and acetic acid (0.750 ml, 13.1 mmol), at 0°C, and stirring was carried out at room temperature for 15 hours. A saturated aqueous sodium hydrogencarbonate solution was added to the reaction solution, followed by extraction with ethyl acetate, and the extract was washed sequentially with water and saline, and dried over anhydrous sodium sulfate. The organic layer was concentrated and the resulting residue was washed with diisopropyl ether, followed by drying to afford t-butyl 4-[(4-fluorophenyl)amino]piperidine-1-carboxylate (3.09 g, yield 80%) as a white solid. 1H-NMR (CDCl3, 400 MHz) δ: 6.91-6.84 (2H, m), 6.56-6.51 (2H, m), 4.12-3.97 (2H, m), 3.36-3.32 (2H, m),2.95-2.87 (1H, m), 2.06-1.98 (2H, m), 1.47 (9H, s), 1.36-1.24 (2H, m).
References:
EP2258697,2010,A1 Location in patent:Page/Page column 50
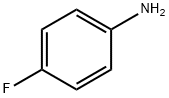
371-40-4
405 suppliers
$5.00/5G

288573-56-8
0 suppliers
inquiry