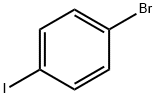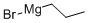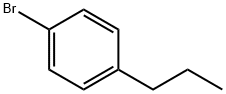
1-Bromo-4-propylbenzene synthesis
- Product Name:1-Bromo-4-propylbenzene
- CAS Number:588-93-2
- Molecular formula:C9H11Br
- Molecular Weight:199.09
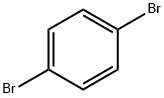
106-37-6

106-94-5

588-93-2
General procedure: 236 g of 1,4-dibromobenzene, 185 g of bromopropane and 1 liter of anhydrous tetrahydrofuran were added to a 2-liter three-neck flask under nitrogen protection. Subsequently, 204 g of zinc chloride, 36 g of magnesium powder and 18 g of iron acetylacetonate were added as catalyst. The reaction mixture was heated to about 40°C under stirring conditions and the reaction started immediately. The reaction temperature was maintained at no more than 60°C for 8 hours. Upon completion of the reaction, the mixture was cooled to room temperature and the reaction was quenched by the addition of 200 ml of water. The tetrahydrofuran solvent was recovered by distillation and the residue was diluted with 500 mL of toluene. The toluene layer was separated, washed twice sequentially with 200 mL of saturated brine and dried over anhydrous sodium sulfate. The dried solution was filtered and the filtrate recovered toluene by distillation. Finally, the 94-97 °C/10 mmHg fraction was collected by vacuum distillation to give 170 g of 4-propylbromobenzene in 85% yield.
Yield:588-93-2 90.5%
Reaction Conditions:
with iron(III)-acetylacetonate;magnesium;zinc(II) chloride in tetrahydrofuran at 40 - 60; for 8 h;Inert atmosphere;
Steps:
5 Example 1
General procedure: Under a nitrogen atmosphere, to a two-liter three-necked flask was added 236 g of p-dibromobenzene, 185 g of 1-bromopropane and 1 liter of anhydrous tetrahydrofuran; followed by addition of 204 g of zinc chloride, 36 g of magnesium powder and 18 Grams of acetylacetone iron. The mixture was heated to about 40 °C under stirring, the reaction was initiated after a few minutes, the reaction temperature was controlled at not more than 60 °C for 8 hours. The reaction mixture was then cooled to room temperature and quenched by stirring with 200 ml of water. The tetrahydrofuran solvent was recovered by distillation and the residue was diluted with 500 ml of toluene. The toluene layer was separated and washed twice with 200 ml of saturated brine and dried over anhydrous sodium sulfate Drying, filtration, filtrate recovery of toluene recovery, and then vacuum distillation, collecting at 94-97 °C / 10 mmHg to give 170 g of p-bromopyrophenyl. The yield was 85%.
References:
East China Normal University;East China University of Science and Technology;Zou Gang;Tang Jie;Wang Zhicheng;Luo Tian;Liu Ting;Guan Changwei;Wang Chen CN106278811, 2017, A Location in patent:Paragraph 0035-0047
10342-83-3
224 suppliers
$5.00/5g

588-93-2
260 suppliers
$9.00/5g
2294-43-1
42 suppliers
$256.00/1g

588-93-2
260 suppliers
$9.00/5g