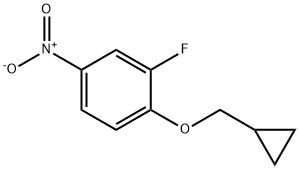
1-(CyclopropylMethoxy)-2-fluoro-4-nitrobenzene synthesis
- Product Name:1-(CyclopropylMethoxy)-2-fluoro-4-nitrobenzene
- CAS Number:1236764-33-2
- Molecular formula:C10H10FNO3
- Molecular Weight:211.19
403-19-0
275 suppliers
$6.00/5g

7051-34-5
407 suppliers
$6.00/1g

1236764-33-2
12 suppliers
$45.00/100mg
Yield:1236764-33-2 13.8 g
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 70; for 0.5 h;
Steps:
18.B B) 1-(cyclopropylmethoxy)-2-fluoro-4-nitrobenzene
B)
1-(cyclopropylmethoxy)-2-fluoro-4-nitrobenzene
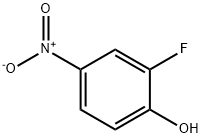
To a solution of 2-fluoro-4-nitrophenol (10.0 g) in DMF (100 mL) were added potassium carbonate (17.6 g) and (bromomethyl)cyclopropane (9.26 mL), and the mixture was stirred with heating at 70° C. for 30 min.
The reaction mixture was allowed to cool to room temperature, diluted with ethyl acetate, and washed with saturated brine.
The obtained organic layer was subjected to silica gel column chromatography (NH, ethyl acetate), and the solvent was evaporated to give the title compound (13.8 g).
1H NMR (300 MHz, CDCl3) δ 0.32-0.51 (2H, m), 0.62-0.81 (2H, m), 1.27-1.42 (1H, m), 3.99 (2H, d, J=7.2 Hz), 7.00 (1H, t, J=8.5 Hz), 7.95-8.08 (2H, m).
References:
US2014/243310,2014,A1 Location in patent:Paragraph 1018; 1019