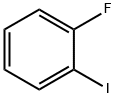
1-Fluoro-2-iodobenzene synthesis
- Product Name:1-Fluoro-2-iodobenzene
- CAS Number:348-52-7
- Molecular formula:C6H4FI
- Molecular Weight:222
445-29-4

348-52-7
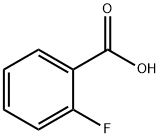
Cesium carbonate (Cs2CO3) (0.6 mmol, 195 mg), o-fluorobenzoic acid (1) (0.3 mmol), photocatalyst [Ir(dF(CF3)ppy)2dtbbpy]PF6(D) (6 μmol, 6.7 mg), N-iodosuccinimide (NIS) (0.9 mmol, 202.5 mg) and iodine (I2) (15 μmol, 5 mol%) were added in order to a 15 mL test tube. 202.5 mg) and iodine (I2) (15 μmol, 5 mol%). The tubes were evacuated and displaced three times with argon, followed by the addition of 3 mL of anhydrous 1,2-dichloroethane (DCE) via syringe under argon protection. The tubes were sealed with Parafilm M, placed in an oil bath fitted with a contact thermometer, and the reaction was carried out at 50 °C by irradiation with a 6 × 5 W blue LED (λmax = 455 nm). After 24 or 36 h of reaction, the reaction mixture was filtered through a 2 cm thick pad of silica gel and the silica gel was washed with dichloromethane (DCM) (50 mL). The filtrates were combined and concentrated under reduced pressure to remove the solvent. The crude product was purified by rapid column chromatography on silica gel to afford the target product o-fluoroiodobenzene (2). (Note: The reaction is extremely sensitive to moisture, and the product yield was significantly reduced to less than 5% when 0.01 equivalents of water was added to the system.)

445-29-4
436 suppliers
$5.00/10g

348-52-7
307 suppliers
$6.00/5g
Yield:348-52-7 64%
Reaction Conditions:
with N-iodo-succinimide;[4,4’-bis(1,1-dimethylethyl)-2,2’-bipyridine-N1,N1‘]bis [3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]iridium(III) hexafluorophosphate;iodine;caesium carbonate in 1,2-dichloro-ethane at 50; for 24 h;Inert atmosphere;Irradiation;Sealed tube;
Steps:
General procedure A.
General procedure: To a 15 mL test tube with septum Cs2CO3 (0.6 mmol, 195 mg), aromaticcarboxylic acid (1) (0.3 mmol), [Ir(dF(CF3)ppy)2dtbbpy]PF6 (D) (6 μmmol, 6.7 mg), Niodosuccinimide(NIS) (0.9 mmol, 202.5 mg) and I2 (15 μmol, 5 mol%) were added. The tube was evacuated and backfilled with argon for three times, and then 3 mL of dry 1,2-dichloroethane(DCE) was added through a syringer under argon. The tube was sealed with Parafilm M andplaced in an oil bath with a contact thermometer, and the reaction was carried out at 50 °C underirradiation with 6 × 5 W blue LEDs (λmax = 455 nm). After 24 or 36 h, the resulting mixture wasfiltered through a 2 cm thick pad of silica, and the silica was washed with dichloromethane (DCM)(50 mL). The filtrate was collected and the solvent was removed in vacuo. The crude residue waspurified by silica gel flash column chromatography to provide the target product (2). (Note: Thereaction was very sensitive to moisture, and the yields sharply decreased to less than 5% when0.01 equivalent of H2O was added to the reaction system).
References:
Jiang, Min;Yang, Haijun;Jin, Yunhe;Ou, Lunyu;Fu, Hua [Synlett,2018,vol. 29,# 12,p. 1572 - 1577] Location in patent:supporting information
348-54-9
431 suppliers
$10.00/1g

348-52-7
307 suppliers
$6.00/5g
462-06-6
432 suppliers
$10.00/5g

348-52-7
307 suppliers
$6.00/5g
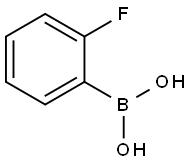
1993-03-9
354 suppliers
$8.00/1g

348-52-7
307 suppliers
$6.00/5g
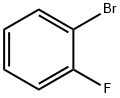
1072-85-1
467 suppliers
$5.00/10g

348-52-7
307 suppliers
$6.00/5g