1-METHYL-1H-PYRROLO[3,2-C]PYRIDINE synthesis
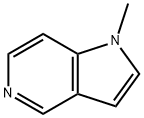
- Product Name:1-METHYL-1H-PYRROLO[3,2-C]PYRIDINE
- CAS Number:24331-97-3
- Molecular formula:C8H8N2
- Molecular Weight:132.16
Yield:24331-97-3 85.78%
Reaction Conditions:
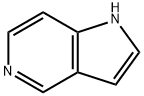
Stage #1: 5-azaindolewith sodium hydride in N,N-dimethyl-formamide; for 0.5 h;Cooling with ice;Inert atmosphere;
Stage #2: iodomethane in N,N-dimethyl-formamide at 20; for 1.5 h;Cooling with ice;Inert atmosphere;
Steps:
82.1; 88.1 step 1):
Compound 1 (500 mg, 4.23 mmol) was dissolved in N-N-dimethylformamide (10 mL), sodium hydride (304.56 mg, 12.7 mmol) was added under ice bath conditions,Then nitrogen was replaced three times, and the mixture was stirred under an ice bath for 30 minutes. Then, methyl iodide (723.89 mg, 5.1 mmol) was slowly injected under an ice bath, and the mixture was stirred at room temperature for 90 minutes.A new spot appeared on the spot plate, diluted with water (20 mL), concentrated under reduced pressure to remove the organic solvent, and the crude product was filtered with methanol,The filtrate was concentrated under reduced pressure to remove the organic solvent, and purified by thin layer chromatography (methanol:dichloromethane=10%) to obtain compound 2 (479 mg). Yield: 85.78%.
References:
WO2022/127923,2022,A1 Location in patent:Page/Page column 156; 157; 161; 162
![4-CHLORO-1-METHYL-1H-PYRROLO[3,2-C]PYRIDINE](/CAS/GIF/27382-01-0.gif)
27382-01-0
67 suppliers
$50.00/10mg
![1-METHYL-1H-PYRROLO[3,2-C]PYRIDINE](/CAS/20180629/GIF/24331-97-3.gif)
24331-97-3
18 suppliers
inquiry