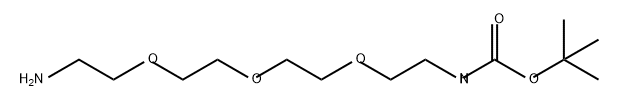
5,8,11-Trioxa-2-azatridecanoic,13-amino,1,1-dimethylethyl ester synthesis
- Product Name:5,8,11-Trioxa-2-azatridecanoic,13-amino,1,1-dimethylethyl ester
- CAS Number:101187-40-0
- Molecular formula:C13H28N2O5
- Molecular Weight:292.37

929-75-9

24424-99-5

101187-40-0
General procedure: 3,6,9-Trioxaundecane-1,11-diamine (1 eq.) was dissolved in dichloromethane (DCM, 10 mL/mmol), and di-tert-butyl dicarbonate (Boc2O, 0.15 eq.) was slowly added at 0 °C. The reaction mixture was stirred at 0°C for 5 hours, then brought to room temperature and continued stirring for 18 hours. Upon completion of the reaction, the organic phase was washed several times with water to remove unreacted 3,6,9-trioxaundecane-1,11-diamine. The organic phase was dried over anhydrous magnesium sulfate (MgSO4) and concentrated under reduced pressure to quantitatively yield the Boc-protected product (tert-butyl 2-(2-(2-(2-aminoethoxy)ethoxy)ethoxy)ethyl)carbamate. 1H NMR (300 MHz, CDCl3) data for product 11a: δ 1.45 (s, 9H), 1.80 (br m, 2H, NH2), 3.25 (m, 2H), 3.38 (m, 2H), 3.50-3.70 (m, 8H), 5.10 (br s, 1H, NHCO2). 1H NMR (300 MHz, CDCl3) data for product 11b: δ 1.42 (s, 9H), 1.75 (br m, 2H, NH2), 2.84 (t, 2H, J = 5.3 Hz), 3.29 (m, 2H), 3.51 (m, 4H), 3.58 (sharp m, 8H), 5.30 (br s, 1H, NHCO2). NHCO2).

929-75-9
176 suppliers
$34.00/250mg

24424-99-5
868 suppliers
$13.50/25G

101187-40-0
190 suppliers
$27.00/100mg
Yield:101187-40-0 100%
Reaction Conditions:
in dichloromethane at 0 - 20; for 23 h;Inert atmosphere;
Steps:
1.1. Mono-protection of 10
General procedure: The diamine 10 (1 equiv) in DCM solution (10 mL / mmol) was treated with Boc2O in default (0.15 equiv) for 5 h at 0 °C and 18 h at room temperature. The organic phase was washed with water, until all the unreacted 10 was extracted. The Boc-protected compound 11 was quantitatively recovered after drying (MgSO4) and concentration under vacuum. 11a: 1H NMR (300 MHz, CDCl3): d 1.45 (s, 9H), 1.80 (br m, 2H, NH2), 3.25 (m, 2H), 3.38 (m, 2H), 3.50-3.70 (m, 8H), 5.10 (br s, 1H, NHCO2). 11b: 1H NMR (300 MHz, CDCl3): d 1.42 (s, 9H), 1.75 (br m, 2H, NH2), 2.84 (t, 2H, J = 5.3 Hz), 3.29 (m, 2H), 3.51 (m, 4H), 3.58 (sharp m, 8H), 5.30 (br s, 1H, NHCO2).
References:
Favre, Anna?ck;Grugier, Jér?me;Brans, Alain;Joris, Bernard;Marchand-Brynaert, Jacqueline [Tetrahedron,2012,vol. 68,# 52,p. 10818 - 10826] Location in patent:supporting information
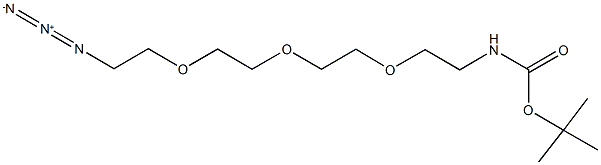
642091-68-7
60 suppliers
inquiry

101187-40-0
190 suppliers
$27.00/100mg

24424-99-5
868 suppliers
$13.50/25G

134179-38-7
219 suppliers
$39.20/100MG

101187-40-0
190 suppliers
$27.00/100mg

929-75-9
176 suppliers
$34.00/250mg
1538-75-6
233 suppliers
$17.67/10gm:

101187-40-0
190 suppliers
$27.00/100mg
![Bis[2-(2-hydroxyethoxy)ethyl] ether](/CAS/GIF/112-60-7.gif)
112-60-7
373 suppliers
$7.00/25g

101187-40-0
190 suppliers
$27.00/100mg