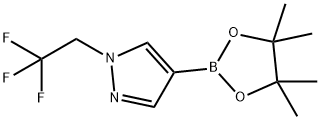
4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1-(2,2,2-trifluoroethyl)-1H-pyrazole synthesis
- Product Name:4-(4,4,5,5-tetraMethyl-1,3,2-dioxaborolan-2-yl)-1-(2,2,2-trifluoroethyl)-1H-pyrazole
- CAS Number:1049730-42-8
- Molecular formula:C11H16BF3N2O2
- Molecular Weight:276.06
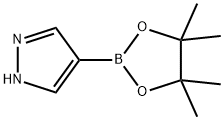
269410-08-4
383 suppliers
$5.00/1g
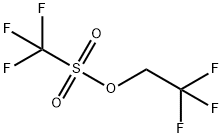
6226-25-1
254 suppliers
$11.19/5G

1049730-42-8
61 suppliers
$68.00/250mg
Yield:1049730-42-8 78%
Reaction Conditions:
with caesium carbonate in N,N-dimethyl-formamide at 20 - 100; for 2 h;
Steps:
255A 255A. Preparation of 4-(4,4,5 ,5-tetramethyl- 1,3 ,2-dioxaborolan-2-yl)- 1 -(2,2,2- trifluoro ethyl)- 1 H-pyrazole
To a stirred solution of 4-(4,4,5 ,5 -tetramethyl- 1,3 ,2-dioxaborolan-2-yl)- 1 Hpyrazole (100 mg, 0.5 15 mmol) in DMF (1 ml) were added Cs2CO3 (252 mg, 0.773mmol) and 2,2,2-trifluoroethyl trifluoromethanesulfonate (0.144 ml, 1.031 mmol) at rt. After stirring at 100 °C for 2 h, the reaction mixture was evaporated to dryness, partitioned between EtOAc and water, and the layers were separated. The organic layer was dried over Na2SO4, filtered, and concentrated to afford 4-(4,4,5,5-tetramethyl-i,3,2- dioxaborolan-2-yl)- 1 -(2,2,2-trifluoroethyl)- 1 H-pyrazole (0.111 g, 78% yield). MS(ESI)m/z: 277.4 (M+H).
References:
BRISTOL-MYERS SQUIBB COMPANY;CORTE, James R.;DE LUCCA, Indawati;FANG, Tianan;YANG, Wu;WANG, Yufeng;DILGER, Andrew K.;PABBISETTY, Kumar Balashanmuga;EWING, William R.;ZHU, Yeheng;WEXLER, Ruth R.;PINTO, Donald J. P.;ORWAT, Michael J.;SMITH, Leon M. II WO2015/116886, 2015, A1 Location in patent:Page/Page column 512

6226-25-1
254 suppliers
$11.19/5G
847818-70-6
118 suppliers
$8.00/100mg

1049730-42-8
61 suppliers
$68.00/250mg

269410-08-4
383 suppliers
$5.00/1g
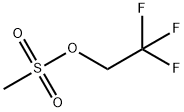
25236-64-0
106 suppliers
$11.19/5G

1049730-42-8
61 suppliers
$68.00/250mg

269410-08-4
383 suppliers
$5.00/1g
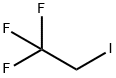
353-83-3
224 suppliers
$11.00/1g

1049730-42-8
61 suppliers
$68.00/250mg