2,2,2-Trifluoroethyl trifluoromethanesulfonate synthesis
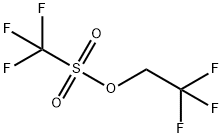
- Product Name:2,2,2-Trifluoroethyl trifluoromethanesulfonate
- CAS Number:6226-25-1
- Molecular formula:C3H2F6O3S
- Molecular Weight:232.1
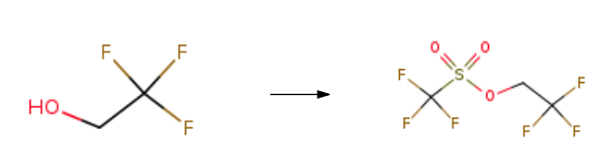
Yield:6226-25-1 95%
Reaction Conditions:
with potassium carbonate in xylene at 25; for 1.2 h;Product distribution / selectivity;Inert atmosphere;
Steps:
5
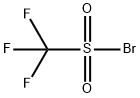
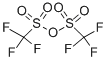
The following are introduced under nitrogen into a 150 ml glass reactor: K2CO3 7 g (50.14 mmol) TFE 3.2 g (32 mmol) solvent 7 g The resulting heterogeneous medium is brought with stirring to a temperature T mentioned in the following table (I) and then TFSBr (7 g, 32 mmol), diluted with TFE (3.2 g, 32 mmol) or with a solvent (7 g), is added over a time t also mentioned in table (I).After the addition, the reaction medium is maintained at the temperature T' for a time t' mentioned in table (I) and then the reactants and the products obtained are quantitatively determined by 19F NMR.The results obtained are recorded in table (I).
References:
US2011/190542,2011,A1 Location in patent:Page/Page column 4
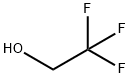
75-89-8
488 suppliers
$9.00/1g

6226-25-1
283 suppliers
$11.19/5G