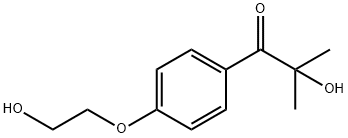
2-Hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone synthesis
- Product Name:2-Hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone
- CAS Number:106797-53-9
- Molecular formula:C12H16O4
- Molecular Weight:224.25
![1-Propanone, 1-[4-(2-hydroxyethoxy)phenyl]-2-methyl-](/CAS/20210111/GIF/159119-01-4.gif)
159119-01-4

106797-53-9
The general procedure for the synthesis of photoinitiator 2959 from the compound (CAS:159119-01-4) was as follows: a 25 mL Schlenk reaction tube was taken and N-bromosuccinimide (NBS) 18 mg (0.1 mmol) was added sequentially as catalyst, 4'-(2-hydroxyethoxy)-2-methylpropiophenone 105 mg (0.5 mmol), and dimethyl sulfoxide (DMSO) 1 mL as oxidizing reagent and solvent. ), and dimethyl sulfoxide (DMSO) 1 mL as oxidizer, carbonylation reagent and solvent. The reaction mixture was stirred at 100 °C for 24 hours. Upon completion of the reaction, 15 mL of ethyl acetate and 3 mL of brine were added to the reaction mixture for extraction, which was then extracted three times with ethyl acetate. All organic phases were combined and purified by column chromatographic separation to give a final purity of 103 mg of 2-hydroxy-4'-(2-hydroxyethoxy)-2-methylpropiophenone in 92% yield.
![1-Propanone, 1-[4-(2-hydroxyethoxy)phenyl]-2-methyl-](/CAS/20210111/GIF/159119-01-4.gif)
159119-01-4
0 suppliers
inquiry

106797-53-9
256 suppliers
$12.00/10g
Yield:106797-53-9 92%
Reaction Conditions:
with N-Bromosuccinimide;dimethyl sulfoxide at 60; for 24 h;Schlenk technique;
Steps:
26 Embodiment 262 - hydroxy -4 ' - (2 - hydroxy ethoxy group) -2 - methylpropiophenone preparation
Taking a 25 ml Schlenk reaction tube, adding N - bromo succimide (NBS) 18 mg (0.1mmol) as catalyst, 4' - (2 - hydroxy ethoxy group) -2 - methylpropiophenone 105 mg (0.5mmol), dimethyl sulfoxide (DMSO) 1 ml as the oxidizing agent, carbonylating and solvent, for 100 °C stirring for 24 hours. After the reaction by adding ethyl acetate 15 ml, salt water 3 ml, ethyl acetate 3 times, the combined organic phase, column chromatography separation to obtain 2 - hydroxy -4 ' - (2 - hydroxy ethoxy group) -2 - methylpropiophenone pure product 103 mg, yield 92%.
References:
CN104710256,2017,B Location in patent:Paragraph 0174-0177
![1-Propanone, 1-[4-[2-(acetyloxy)ethoxy]phenyl]-2-methyl-](/CAS/20200401/GIF/106797-52-8.gif)
106797-52-8
0 suppliers
inquiry

106797-53-9
256 suppliers
$12.00/10g
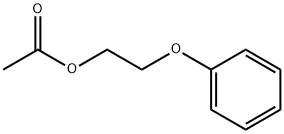
6192-44-5
115 suppliers
$29.00/25g
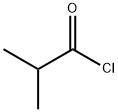
79-30-1
369 suppliers
$10.00/10g

106797-53-9
256 suppliers
$12.00/10g
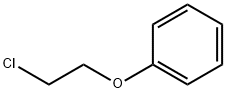
622-86-6
166 suppliers
$5.00/5g

106797-53-9
256 suppliers
$12.00/10g

108-95-2
786 suppliers
$14.00/25g

106797-53-9
256 suppliers
$12.00/10g