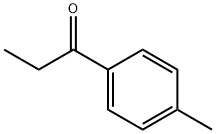
4'-Methylpropiophenone synthesis
- Product Name:4'-Methylpropiophenone
- CAS Number:5337-93-9
- Molecular formula:C10H12O
- Molecular Weight:148.2
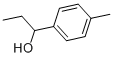
25574-04-3
22 suppliers
$90.00/1g

5337-93-9
491 suppliers
$10.00/25g
Yield:5337-93-9 93%
Reaction Conditions:
with 2,6-dimethylpyridine;ABNO;sodium perchlorate in acetonitrile; for 8.66 h;Inert atmosphere;Electrochemical reaction;
Steps:
3.4. Preparation Electrolysis Experiments
General procedure: The preparative electrolysis experiments were conducted with in an undivided cell containing0.1 M NaClO4-CH3CN solution (15 mL), alcohol substrate (1.0 mmol), ABNO (0.1 mmol),and 2,6-lutidine (1.0 mmol) at a constant current of 10.0 mA with moderate magnetic stirring for8.5 h in the atmosphere. Two square platinum sheets were employed as the anode and cathode,respectively. The electrolytic reaction was monitored by gas chromatography (GC) on a GC-2010system (Shimadzu, Kyoto, Japan) equipped with a SH-Rtx-Was polar column and a flame ionizationdetector (FID). Both the injector and detector were maintained at 220 °C, the carrier gas is nitrogen,and the flow rate is 1.2 mL/min. The initial oven temperature of 100 °C was held for 2 min andthen ramped up at 15 °C per min to 220 °C. This final temperature was held for 8 min. After thereaction was finished, the resulting mixture was concentrated in a rotary evaporator (Heidolph,Schwabach, Germany) and purified by column chromatography on silica gel using petroleum andethyl acetate 15:1) as eluent to afford the products. The products were confirmed by GC-MS, 1H-NMR,and 13C-NMR. NMR spectroscopy was carried out on a Bruker Avance III spectrometer (Bruker,F?llanden, Switzerland). The GC-MS analysis was measured on Thermo Trace ISQ instrument (ThermoFisher Nicolet,Waltham, MA, USA) with TG 5MS capillary column.Acetophenone (colorless oil, yield 80%):
References:
Niu, Pengfei;Liu, Xin;Shen, Zhenlu;Li, Meichao [Molecules,2019,vol. 24,# 1,art. no. 100]
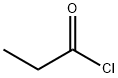
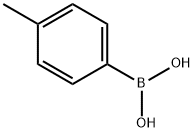
79-03-8
392 suppliers
$9.00/1g

5337-93-9
491 suppliers
$10.00/25g
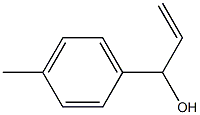
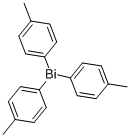
58824-48-9
2 suppliers
inquiry

5337-93-9
491 suppliers
$10.00/25g