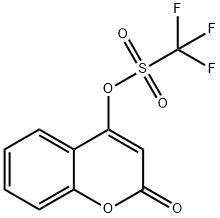
Methanesulfonic acid, 1,1,1-trifluoro-, 2-oxo-2H-1-benzopyran-4-yl ester synthesis
- Product Name:Methanesulfonic acid, 1,1,1-trifluoro-, 2-oxo-2H-1-benzopyran-4-yl ester
- CAS Number:113777-29-0
- Molecular formula:C10H5F3O5S
- Molecular Weight:294.2
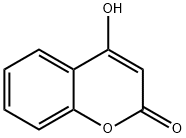
1076-38-6
526 suppliers
$24.00/100g
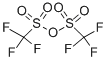
358-23-6
573 suppliers
$10.00/1g

113777-29-0
1 suppliers
inquiry
Yield:113777-29-0 97%
Reaction Conditions:
with triethylamine in dichloromethane at -10; for 2 h;
Steps:
1 Step 1 : 2-oxo-2H-chromen-4-yl trifluoromethanesulfonate (28a)
Step 1 : 2-oxo-2H-chromen-4-yl trifluoromethanesulfonate (28a) 4-Hydroxycoumarine (1 g, 6.17 mmoles, 1 eq.) was dissolved in DCM (30 mL), triethylamine (1 .72 mL, 1 2.34 mmoles, 2 eq.) was added and the resulting mixture was cooled to -I CC. Trifluoromethanesulfonic anhydride (1 .25 mL, 7.4 mmoles, 1 .2 eq.) in DCM (5 mL) was added dropwise and the solution was left stirring at -10°C for 2 h. The resulting reddish brown solution was warmed to room temperature, diluted with cHex/Et20 1 /1 (50 mL) and filtered through a pad of silica gel using cHex/Et20 1 /1 as eluent. The solvent was removed in vacuum to obtain 2-oxo-2H-chromen-4-yl trifluoromethanesulfonate 28a (1 .76 g, Y= 97%). LC-MS (M-H+): 295.0.
References:
WO2016/96631,2016,A1 Location in patent:Page/Page column 31
582-24-1
238 suppliers
$8.00/5g

113777-29-0
1 suppliers
inquiry
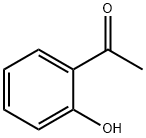
118-93-4
545 suppliers
$10.00/25g

113777-29-0
1 suppliers
inquiry