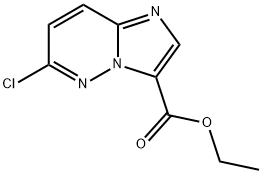
6-Chloro-iMidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester synthesis
- Product Name:6-Chloro-iMidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester
- CAS Number:1150566-27-0
- Molecular formula:C9H8ClN3O2
- Molecular Weight:225.63
![Pyridazinium, 3-chloro-6-[[(dimethylamino)methylene]amino]-1-(2-ethoxy-2-oxoethyl)-, bromide (1:1)](/CAS/20211123/GIF/1150566-26-9.gif)
1150566-26-9
![6-Chloro-iMidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester](/CAS/GIF/1150566-27-0.gif)
1150566-27-0
General procedure for the synthesis of ethyl 6-chloroimidazo[1,2-B]pyridazine-3-carboxylate from the compound (CAS:1150566-26-9): S-chloro-β-dimethylamino-methyleneamino-J-isoethyloxycarbonylmethyl-pyridazine-1-bromide (11.40 g, 32.40 mmol) was dissolved in acetonitrile (200 mL), followed by addition of diisopropylethylamine (15.22 mL, 64.81 mmol). The reaction mixture was stirred at room temperature for 4 hours. After completion of the reaction, the solvent was removed by distillation under reduced pressure and the residue was ground with water to afford 5.77 g of ethyl 6-chloroimidazo[1,2-b]pyridazine-3-carboxylate as a light brown solid in 77% yield. The product was characterized by 1H NMR (300 MHz, CDCl3): δ 8.36 (1H, s), 8.01 (1H, d, J = 9.4 Hz), 7.27 (1H, d, J = 9.4 Hz), 4.46 (2H, q, J = 7.1 Hz), 1.37 (3H, t, J = 7.1 Hz).LCMS analysis showed m/z 226 [M + 1]+ (molecular weight: 225.64).
![Pyridazinium, 3-chloro-6-[[(dimethylamino)methylene]amino]-1-(2-ethoxy-2-oxoethyl)-, bromide (1:1)](/CAS/20211123/GIF/1150566-26-9.gif)
1150566-26-9
0 suppliers
inquiry
![6-Chloro-iMidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester](/CAS/GIF/1150566-27-0.gif)
1150566-27-0
114 suppliers
$45.00/50mg
Yield:1150566-27-0 77%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in acetonitrile at 20; for 4 h;
Steps:
2
Intermediate 2 6-Chloro-imidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester; S-Chloro-β^dimethylamino-methyleneaminoJ-i-ethoxycarbonylmethyl-pyridazin- 1-ium bromide (11.40 g, 32.40 mmol) was dissolved in acetonitrile (200 mL) and diisopropylethylamine (15.22 mL, 64.81 mmol) was added. The reaction mixture was stirred for 4 hours at room temperature. The solvent was removed in vacuo and the residue was triturated from water to give 5.77 g of 6-chloro-imidazo[1 ,2- b]pyridazine-3-carboxylic acid ethyl ester as a pale brown solid (77% yield). 1H NMR (300 MHz, CDCI3): δ 8.36 (1 H, s), 8.01 (1H, d, J = 9.4 Hz), 7.27 (1H, d, J = 9.4 Hz), 4.46 (2H, q, J = 7.1 Hz)1 1.37 (3H, W = 7.1 Hz). LCMS: 226 [M+1J. (MW: 225.64)
References:
WO2009/60197,2009,A1 Location in patent:Page/Page column 59
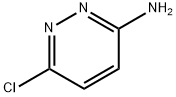
5469-69-2
375 suppliers
$6.00/10g
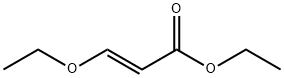
5941-55-9
61 suppliers
inquiry
![6-Chloro-iMidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester](/CAS/GIF/1150566-27-0.gif)
1150566-27-0
114 suppliers
$45.00/50mg

5469-69-2
375 suppliers
$6.00/10g
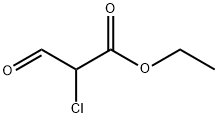
33142-21-1
130 suppliers
$12.00/250mg
![6-Chloro-iMidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester](/CAS/GIF/1150566-27-0.gif)
1150566-27-0
114 suppliers
$45.00/50mg

5469-69-2
375 suppliers
$6.00/10g
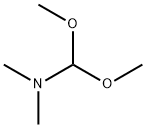
4637-24-5
646 suppliers
$5.00/25g
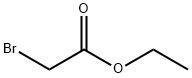
105-36-2
349 suppliers
$5.00/5G
![6-Chloro-iMidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester](/CAS/GIF/1150566-27-0.gif)
1150566-27-0
114 suppliers
$45.00/50mg

105-36-2
349 suppliers
$5.00/5G
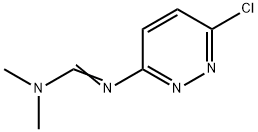
35053-55-5
19 suppliers
inquiry
![6-Chloro-iMidazo[1,2-b]pyridazine-3-carboxylic acid ethyl ester](/CAS/GIF/1150566-27-0.gif)
1150566-27-0
114 suppliers
$45.00/50mg