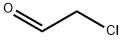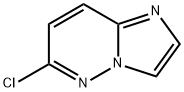
6-Chloroimidazo[2,1-f]pyridazine synthesis
- Product Name:6-Chloroimidazo[2,1-f]pyridazine
- CAS Number:6775-78-6
- Molecular formula:C6H4ClN3
- Molecular Weight:153.57
5469-69-2
2032-35-1
![6-Chloroimidazo[2,1-f]pyridazine](/CAS/GIF/6775-78-6.gif)
6775-78-6
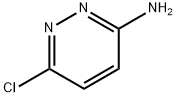
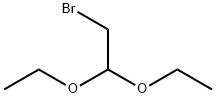
General procedure for the preparation of 6-chloroimidazo[1,2-b]pyridazine: Bromoacetaldehyde diethyl acetal (13.7 g, 69.5 mmol, 1.8 eq.) was added to hydrobromic acid (4.0 mL) and heated to reflux for 1.5 hours. Upon completion of the reaction, the mixture was cooled to room temperature and subsequently poured into an isopropanol reaction flask containing an excess of sodium bicarbonate. After stirring for 3 minutes, filtration was performed. To the filtrate was added 3-amino-6-chloropyridazine (5.0 g, 38.6 mmol, 1.0 eq.) and the reflux was continued by heating for 2 hours. At the end of the reaction, the reaction mixture was quenched with water and extracted with ethyl acetate. Finally, the product was purified by column chromatography to give 5.1 g of brown solid in 86% yield. The product was characterized by 1H NMR (400 MHz, CDCl3): δ 7.89 (s, 1H), 7.88 (d, J = 9.3 Hz, 1H), 7.74 (s, 1H), 7.01 (d, J = 9.3 Hz, 1H).
Yield:6775-78-6 92%
Reaction Conditions:
in water;butan-1-ol at 20; for 18 h;Reflux;
Steps:
6 -Chloroimidazo[1,2-b]pyridazine(1)
Chloroacetaldehyde (9.2 mL, 50% in H2O) wasadded to a solution of 3-amino-6-chloropyridazine (6 g, 46 mmol) in 70 mL n-butanolat room temperature. This mixture was refluxed for 18 h and the then cooled toroom temperature. The resulting precipitate was filtered, washed with butanoland ethyl ether and dissolved in water. NaOH (1N) was added and the extractedwith ethyl acetate and the organic layer was washed with NaHCO3. Theorganic layer was dried over Na2SO4, filtered, andevaporated under reduced pressure to give the compound 1 as a pink amorphous in 92% yield: Mp 116-118 °C; Litt1 115-117.5°C; 1H NMR (CDCl3):δ 7.35 (d, 1H, J = 9.6 Hz, H-8pyridazine),7.85 (s, 1H, H-2Imid),8.22 (d, 1H, J = 9.6 Hz, H-7pyridazine), 8.34 (s, 1H, H-3Imid). 13CNMR (DMSO-d6): δ117.99 (CH), 119.40 (CH), 128.21 (CH), 134.99 (CH), 137.67 (C), 146.78(C).HPLC: Rt= 1.55 min.
References:
Bendjeddou, Lyamin Z.;Loa?c, Nadège;Villiers, Beno?t;Prina, Eric;Sp?th, Gerald F.;Galons, Hervé;Meijer, Laurent;Oumata, Nassima [European Journal of Medicinal Chemistry,2017,vol. 125,p. 696 - 709] Location in patent:supporting information

5469-69-2
375 suppliers
$6.00/10g

2032-35-1
488 suppliers
$5.00/10g
![6-Chloroimidazo[2,1-f]pyridazine](/CAS/GIF/6775-78-6.gif)
6775-78-6
284 suppliers
$6.00/1g

5469-69-2
375 suppliers
$6.00/10g
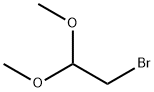
7252-83-7
416 suppliers
$5.00/10g
![6-Chloroimidazo[2,1-f]pyridazine](/CAS/GIF/6775-78-6.gif)
6775-78-6
284 suppliers
$6.00/1g

5469-69-2
375 suppliers
$6.00/10g
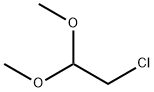
97-97-2
299 suppliers
$18.00/25mL
![6-Chloroimidazo[2,1-f]pyridazine](/CAS/GIF/6775-78-6.gif)
6775-78-6
284 suppliers
$6.00/1g