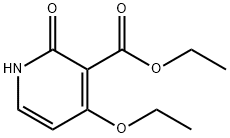
Ethyl 4-Ethoxy-2-oxo-1,2-dihydropyridine-3-carboxylate synthesis
- Product Name:Ethyl 4-Ethoxy-2-oxo-1,2-dihydropyridine-3-carboxylate
- CAS Number:1174046-84-4
- Molecular formula:C10H13NO4
- Molecular Weight:211.21
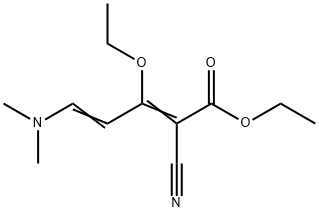
1345855-03-9

1174046-84-4
General procedure for the synthesis of ethyl 2-oxo-4-ethoxy-1,2-dihydropyridine-3-carboxylate from ethyl 2-cyano-5-(dimethylamino)-3-ethoxypenta-2,4-dienoate: ethyl 2-cyano-5-(dimethylamino)-3-ethoxypenta-2,4-dienoate (15.0 g, 63 mmol) was mixed with acetic acid (600 mL) and reacted at reflux overnight. After completion of the reaction, the mixture was cooled to room temperature and concentrated under reduced pressure to remove the solvent. The residue was treated with water and washed with ethyl acetate (2×). The aqueous layer was adjusted to pH 9-10 with sodium bicarbonate and extracted with dichloromethane (3×). The organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate and concentrated under reduced pressure. Finally, it was purified by silica gel column chromatography to afford ethyl 2-oxo-4-ethoxy-1,2-dihydropyridine-3-carboxylate (9.0 g, 67% yield).

1345855-03-9
9 suppliers
inquiry

1174046-84-4
73 suppliers
$45.00/25mg
Yield: 67%
Reaction Conditions:
with acetic acidReflux;
Steps:
A4
[01 03j A mixture of ethyl 2-cyano-5 -(dimethylamino)-3 -ethoxypenta-2,4-dienoate (15 Og, 0.63 mol) and HOAc (600 mL) was refluxed overnight. The mixture was cooled to RT, concentrated to dryness, treated with water and washed with EtOAc (2x). The aqueous layer was made basic (pH=9-10) with NaHCO3, extracted with DCM (3x) and the combined organics were washed with brine, dried over Na2SO4, concentrated and purified by silica gel chromatography to afford ethyl 4-ethoxy-2-oxo- 1 ,2-dihydropyridine-3 -carboxylate (90 g, 67% yield).
References:
DECIPHERA PHARMACEUTICALS, LLC;FLYNN, Daniel L.;KAUFMAN, Michael D. WO2014/145004, 2014, A1 Location in patent:Paragraph 0103
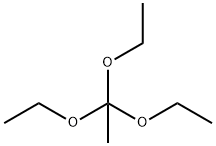
78-39-7
374 suppliers
$9.00/1g

105-56-6
461 suppliers
$10.00/5ml
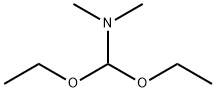
1188-33-6
272 suppliers
$11.19/5ML

1174046-84-4
73 suppliers
$45.00/25mg

78-39-7
374 suppliers
$9.00/1g

105-56-6
461 suppliers
$10.00/5ml

1174046-84-4
73 suppliers
$45.00/25mg

105-56-6
461 suppliers
$10.00/5ml

1174046-84-4
73 suppliers
$45.00/25mg
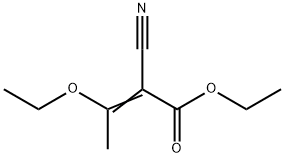
35260-93-6
33 suppliers
inquiry

1174046-84-4
73 suppliers
$45.00/25mg