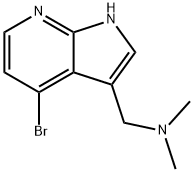
1H-Pyrrolo[2,3-b]pyridine-3-MethanaMine, 4-broMo-N,N-diMethyl- synthesis
- Product Name:1H-Pyrrolo[2,3-b]pyridine-3-MethanaMine, 4-broMo-N,N-diMethyl-
- CAS Number:1198277-82-5
- Molecular formula:C10H12BrN3
- Molecular Weight:254.13

50-00-0
896 suppliers
$10.00/25g
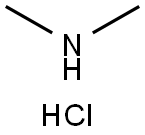
506-59-2
557 suppliers
$5.00/5G
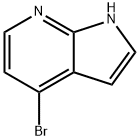
348640-06-2
338 suppliers
$6.00/250mg
![1H-Pyrrolo[2,3-b]pyridine-3-MethanaMine, 4-broMo-N,N-diMethyl-](/CAS/GIF/1198277-82-5.gif)
1198277-82-5
4 suppliers
inquiry
Yield:1198277-82-5 42%
Reaction Conditions:
Stage #1: formaldehyd;N,N-dimethylammonium chloride;4-bromo-7-azaindole in butan-1-ol at 120; for 1.25 h;
Stage #2: with potassium carbonate in water;Saturated solution;
Steps:
Preparation of 1-methyl-4-phenyl-1H-pyrrolo[2,3-b]pyridine-3-carbaldehyde (Formula 17); A solution of 70% mCPBA (11.54 g, 66.87 mmole) in ethyl acetate (25 mL) was added by drops to a solution of 7-azaindole (Formula 11, 5 g, 42.3 mmole) in ethyl acetate (40 mL) at 0° C. with a good stirrer. After addition was completed, the mixture was stirred at room temperature for 1 to 2 hours until no starting material left. The mixture was cooled, filtered, and washed with ethyl acetate to give a solid. It was dissolved in 50 mL of water and treated with 30% K2CO3 solution (16 mL) to pH 9.5-10.5 to give a precipitate. It was stirred at room temperature for 1 hour, cooled, filtered, and washed with a small amount of cold water to give 2.484 g of 1H-pyrrolo[2,3-b]pyridine 7-oxide as a white crystal (Formula 12, 43.8% yield). MS (m/z): 135.1 (MH+).A solution of methanesulfonic anhydride (6.066 g, 34.82 mmole) and acetonitrile (11.7 mL) was added by drops to a solution of 1H-pyrrolo[2,3-b]pyridine 7-oxide (Formula 12, 2.333 g, 17.41 mmole), tetramethyl ammonium bromide (4.023 g, 26.12 mmole) in DMF (11.7 mL) at 0° C. After stirring at 0° C. for 45 min, additional DMF (11.7 mL) was added in drops to the thick mixture at 0° C., and then stirred at room temperature overnight. To the mixture was added ice water (35 mL), followed by 10 N NaOH (4.66 mL) to pH 7. After stirring at the room temperature, a precipitate formed. It was filtered and washed with water to give 1.891 g of 4-bromo-1H-pyrrolo[2,3-b]pyridine as a pale peach solid (Formula 13, 55% yield). MS (m/z): 197 (MH+). NMR (DMSO-d6) showed 69% impurity which is likely to be the 4,6-dibromo compound based on LC/MS analysis.A mixture of 4-bromo-1H-pyrrolo[2,3-b]pyridine (Formula 13, 197 mg, 1 mmole), dimethylamine hydrochloride (88 mg, 1.079 mmole), and paraformaldehyde (33 mg, 1.1 mmole) in n-butanol (2 mL) was heated at 120° C. for 1.25 hours. After removal of the solvent, the residue was treated with ice water and three drops of con. HCl. After washing with ether, the aqueous layer was basified with sat. K2CO3 solution and extracted with methylene chloride. The organic layer was dried over sodium sulfate, filtered, and the solvent dried to give 106 mg of 1-(4-bromo-1H-pyrrolo[2,3-b]pyridin-3-yl)-N,N-dimethylmethanamine as a light pink solid (Formula 14, 42%). MS (m/z): 254.2 (MH+).A solution of 1-(4-bromo-1H-pyrrolo[2,3-b]pyridin-3-yl)-N,N-dimethylmethanamine (Formula 14, 341 mg, 1.34 mmole) and hexamethylenetetramine (190 mg, 1.34 mmole) in 66% propionic acid was added by drops to a refluxing solution of hexamethylenetetramine (190 mg, 1.34 mmole) in 66% propionic acid (0.8 mL) at 120° C. The reaction mixture was heated for 2-4 hours, and monitored by MS. It was cooled, treated with water (4 mL), and filtered to give 238 mg of 4-bromo-1H-pyrrolo[2,3-b]pyridine-3-carbaldehyde as a beige solid (Formula 15, 79%). MS (m/z): 225.0 (MH+).Sodium hydride (60%, 27.4 mg, 0.686 mmole) was added in portions to a suspension of 4-bromo-1H-pyrrolo[2,3-b]pyridine-3-carbaldehyde (Formula 15, 128.6 mg, 0.572 mmole) in 5 mL of DMF and 1 mL of THF at 0° C. After stirring at 0° C. for 20 min, methyl iodide (39.2 μL, 0.6292 mmole) was added by drops into the mixture and warmed up to room temperature for 2.5 hours. The solvents were evaporated and the residue was treated with methylene chloride, filtered, and dried. This was further treated with hexane. The mixture was filtered again and washed with hexane to give a beige solid, which was recrystallized from chloroform and hexane to yield 102 mg of 4-bromo-1-methyl-1H-pyrrolo[2,3-b]pyridine-3-carbaldehyde as crystals (Formula 16, 74%). MS (ESI): m/z 239 (M+H).A mixture of 4-bromo-1-methyl-1H-pyrrolo[2,3-b]pyridine-3-carbaldehyde (Formula 16, 38 mg, 0.159 mmole), phenyl boronic acid (38.8 mg, 0.318 mmole), and tetrakis(triphenylphosphine)palladium (0) (27.6 mg, 0.0238 mmole) in saturated sodium carbonate (0.37 mL) and 1,2-dimethoxylethane (1.4 mL) was heated at 120° C. in microwave for 20 min. It was filtered through a pad of silica gel and washed with 5% MeOH in ethyl acetate. After the solvent was evaporated, acetonitrile was added to the residue, and filtered to remove a bright yellow solid. The filtrate was concentrated to yield 51.4 mg of 1-methyl-4-phenyl-1H-pyrrolo[2,3-b]pyridine-3-carbaldehyde as white crystals (Formula 17, Ar=phenyl, 76%). MS (ESI): m/z 237(M+H).
References:
US2009/298820,2009,A1 Location in patent:Page/Page column 36-37