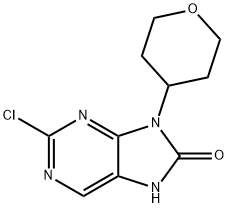
2-chloro-7-methyl-9-(tetrahydro-2H-pyran-4-yl)-7,9-dihydro-8H-purin-8-one synthesis
- Product Name:2-chloro-7-methyl-9-(tetrahydro-2H-pyran-4-yl)-7,9-dihydro-8H-purin-8-one
- CAS Number:1299420-90-8
- Molecular formula:C11H13ClN4O2
- Molecular Weight:268.7
Yield:1299420-90-8 90%
Reaction Conditions:
Stage #1: 2-chloro-9-(tetrahydro-2H-pyran-4-yl)-7,9-dihydro-8H-purin-8-onewith sodium hydride in N,N-dimethyl-formamide at 0;
Stage #2: methyl iodide in N,N-dimethyl-formamide at 20;
Steps:
1.d Step d: Preparation of Intermediate M4
Under the condition of 0 °C, to the N,N-dimethylformamide solution (15 mL) of M3 (6.3 g, 1 eq), NaH (1.97 g, 2 eq) was slowly added in batches, and stirred at 0 °C for 20-30 min. Add iodomethane (4.62mL, 3eq) in N,N-dimethylformamide solution (5mL), slowly return the temperature to room temperature, after TLC shows that the reaction is over, slowly add a small amount of saturated brine under ice bath, wait for the mixture After no more bubbles were generated, a large amount of saturated brine was added, and a large amount of solid was precipitated. After suction filtration, the obtained solid was vacuum-dried overnight to obtain M4, white powder, yield 90%
References:
WO2022/199547,2022,A1 Location in patent:Page/Page column 19-21
33024-60-1
205 suppliers
$6.00/1g

1299420-90-8
10 suppliers
inquiry

899806-21-4
4 suppliers
inquiry

1299420-90-8
10 suppliers
inquiry

1299420-86-2
0 suppliers
inquiry

1299420-90-8
10 suppliers
inquiry