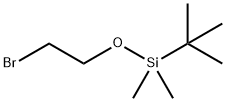
2-[[2-[[(1,1-Dimethylethyl)dimethylsilyl]oxy]ethyl]amino]ethanol synthesis
- Product Name:2-[[2-[[(1,1-Dimethylethyl)dimethylsilyl]oxy]ethyl]amino]ethanol
- CAS Number:1402005-01-9
- Molecular formula:C10H25NO2Si
- Molecular Weight:219.3965
Yield:1402005-01-9 100%
Reaction Conditions:
in acetonitrile; for 3 h;Reflux;
Steps:
13; 6.1 Step 1. Preparation of 2-((2-((tert-butyldimethylsilyl)oxy)ethyl)amino)ethan-l-ol 51
A solution of ethanolamine (77 mL, 1.25 mol) and (2-bromoethoxy)-tert-butyl dimethylsilane (15 g, 62.7 mmol) in anhydrous acetonitrile (200 mL) was refluxed for 3 hours. Upon completion the reaction was cooled to room temperature, diluted with water (400 mL) and extracted with ethyl acetate (3 x 150 mL). The combined ethyl acetate extracts were dried on magnesium sulfate, filtered and concentrated in vacuo to dryness. The residue was purified by filtration through a pad of silica first with 50% ethyl acetate/hexanes then 50% MeOH/EtOAc to afford 51 as a pale yellow oil (14 g, 100%).
References:
WO2017/177326,2017,A1 Location in patent:Page/Page column 79-81

18162-48-6
681 suppliers
$9.00/5g
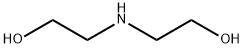
111-42-2
865 suppliers
$10.00/20mg
![2-[[2-[[(1,1-Dimethylethyl)dimethylsilyl]oxy]ethyl]amino]ethanol](/CAS/20200331/GIF/CB45376715.gif)
1402005-01-9
6 suppliers
inquiry
102191-92-4
150 suppliers
$10.00/250mg

141-43-5
913 suppliers
$9.00/10g
![2-[[2-[[(1,1-Dimethylethyl)dimethylsilyl]oxy]ethyl]amino]ethanol](/CAS/20200331/GIF/CB45376715.gif)
1402005-01-9
6 suppliers
inquiry

18162-48-6
681 suppliers
$9.00/5g
![2-[[2-[[(1,1-Dimethylethyl)dimethylsilyl]oxy]ethyl]amino]ethanol](/CAS/20200331/GIF/CB45376715.gif)
1402005-01-9
6 suppliers
inquiry