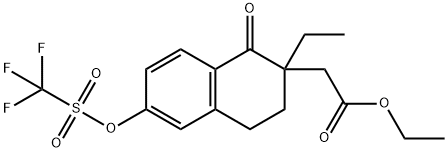
Ethyl 2-(2-ethyl-1-oxo-6-(((trifluoromethyl)sulfonyl)oxy)-1,2,3,4-tetrahydronaphthalen-2-yl)acetate synthesis
- Product Name:Ethyl 2-(2-ethyl-1-oxo-6-(((trifluoromethyl)sulfonyl)oxy)-1,2,3,4-tetrahydronaphthalen-2-yl)acetate
- CAS Number:1414797-96-8
- Molecular formula:C17H19F3O6S
- Molecular Weight:408.39
Yield:1414797-96-8 67.7%
Reaction Conditions:
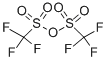
with pyridine in dichloromethane at 20; for 2 h;Cooling with ice;
Steps:
4 Ethyl 2-(2-ethyl-l-oxo-6-(trifluoromethylsulfonyloxy)-l,2,3,4-tetrahvdronaphthalen-2-yl) acetate (4D):
Ethyl 2-(2-ethyl-l-oxo-6-(trifluoromethylsulfonyloxy)-l,2,3,4-tetrahvdronaphthalen-2-yl) acetate (4D): 4D Trifluoroacetic anhydride (3.67 mL, 21.71 mmol) was added to an ice cold solution of 4C (4 g, 14.48 mmol) and pyridine (1.756 mL, 21.71 mmol) in dichloromethane (50 mL), and the mixture was stirred at room temperature for 2 h. The reaction mixture was diluted with dichloromethane (100 mL) and extracted with saturated aqueous solution of NaCl (75 mL). The organic layer was dried over sodium sulfate and filtered, and the filtrate was concentrated under vacuum. The crude product was purified by flash chromatography using 5% ethyl acetate in hexanes to afford the title compound (4 g, 67.7%) as a syrup. lU NMR (300 MHz, CDC13): δ 8.16 (d, J= 8.7 Hz, 1H), 7.23 - 7.12 (m, 2H), 4.09 (q, J= 7.2 Hz, 2H), 3.2 - 2.85 (m, 3H), 2.58 - 2.4 (m, 2H), 2.06 (dt, J = 9.6 Hz, J2 = 4.2 Hz, 1H), 1.8 - 1.55 (m, 2H), 1.21 (t, J= 7.2 Hz, 3H), 0.92 (t, J= 7.5 Hz, 3H). ESI-MS m/z = 409 (M+H)+.
References:
WO2014/74761,2014,A2 Location in patent:Page/Page column 42
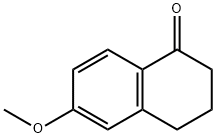
1078-19-9
380 suppliers
$6.00/5g

1414797-96-8
1 suppliers
inquiry

50558-96-8
3 suppliers
inquiry

1414797-96-8
1 suppliers
inquiry

1414797-93-5
1 suppliers
inquiry

1414797-96-8
1 suppliers
inquiry
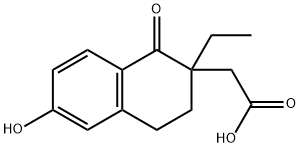
1414797-94-6
1 suppliers
inquiry

1414797-96-8
1 suppliers
inquiry