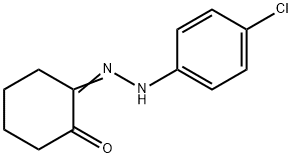
1,2-CYCLOHEXANEDIONE, MONO[(4-CHLOROPHENYL)HYDRAZONE] synthesis
- Product Name:1,2-CYCLOHEXANEDIONE, MONO[(4-CHLOROPHENYL)HYDRAZONE]
- CAS Number:14192-45-1
- Molecular formula:C12H13ClN2O
- Molecular Weight:236.7
Yield:14192-45-1 49%
Reaction Conditions:
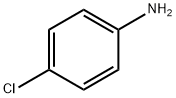
Stage #1: 4-chloro-anilinewith hydrogenchloride;sodium nitrite in water at 0; for 0.833333 h;
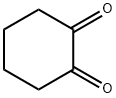
Stage #2: 2-(hydroxymethylene)cyclohexanonewith sodium acetate in methanol;water at 0; for 0.583333 h;
Steps:
1; a Example 1: 6-Chloro-2.3.4.9-tetrahydro-1 H-carbazol-1 -one. a) Cyclohexane-1,2-dione (4-chlorophenyI)hydrazone.
To a cold (0C) solutionof 4-chloroaniline (5.6 g, 44 mmol) in concentrated hydrochloric acid (5 ml_) wasadded sodium nitrite (3.0 g, 44 mmol) dissolved in water (10 mL) portionwise over 20minutes. The mixture was stirred at 0C for 30 minutes. In a separate flask, a coolsolution of 2-(hydroxymethylene)cyclohexanone (Organic Syntheses, Collective Vol4, 1963, pg. 536) (5.0 g, 40 mmol) in methanol (30 mL) was treated with a solution ofsodium acetate (8.3 g, 101 mmol) in water (25 mL). The mixture was stirred at 0Cfor 20 minutes and the diazonium salt slurry was added. The combined mixture wasstirred for 1015 minutes, collected by filtration, triturated with ethanol, and collectedby filtration to give cyclohexane-1,2-dione (4-chlorophenyl)hydrazone (4.6 g, 49%yield) as a yellow solid. 1H-NMR (DMSO-c/6): 5 9.93 (s, 1H), 7.29 (m, 4H), 2.55 (m,2H), 2.40 (m, 2H), 1.84-1.75 (m, 4H).
References:
WO2004/110999,2004,A1 Location in patent:Page 27-28
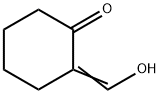
823-45-0
8 suppliers
inquiry
![1,2-CYCLOHEXANEDIONE, MONO[(4-CHLOROPHENYL)HYDRAZONE]](/CAS/GIF/14192-45-1.gif)
14192-45-1
5 suppliers
inquiry