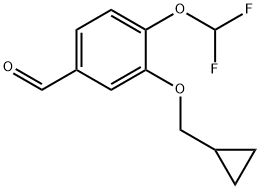
4-(DIFLUOROMETHOXY)-3-(CYCLOPROPYLMETHOXY)-BENZALDEHYDE synthesis
- Product Name:4-(DIFLUOROMETHOXY)-3-(CYCLOPROPYLMETHOXY)-BENZALDEHYDE
- CAS Number:151103-09-2
- Molecular formula:C12H12F2O3
- Molecular Weight:242.22

7051-34-5
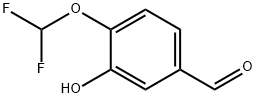
151103-08-1

151103-09-2
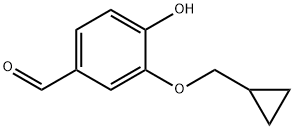
The general procedure for the synthesis of 4-(difluoromethoxy)-3-(cyclopropylmethoxy)benzaldehyde from bromomethylcyclopropane and 4-difluoromethoxy-3-hydroxybenzaldehyde was as follows: 55 g of 4-(difluoromethoxy)-3-hydroxybenzaldehyde, 42.42 g of K2CO3 (1.05 eq.), 4.86 g of KI (0.1 eq.), and 220 mL of dimethyl sulfoxide ( DMSO) were added to the reactor. The mixture was heated and stirred at 70 °C for 1 hour. Subsequently, a pre-prepared mixture of 42.65 g of bromomethylcyclopropane (1.08 eq.) and 110 mL of DMSO was slowly added dropwise for a controlled time of 1 hour. After the dropwise addition was completed, the reaction was continued at 70 °C for 3 hours. Upon completion of the reaction, the reaction mixture was cooled to room temperature. The reaction mixture was diluted by adding 375 mL of toluene and filtered to remove unreacted K2CO3. The filtrate was cooled to 0-5 °C and 375 mL of deionized water was added. The organic and aqueous phases were separated and the organic phase was washed twice with 55 mL of deionized water. Finally, the solvent was removed by distillation under reduced pressure to give 70 g (99% yield) of 4-(difluoromethoxy)-3-(cyclopropylmethoxy)benzaldehyde as a viscous pale yellow liquid.

7051-34-5
410 suppliers
$6.00/1g

151103-08-1
275 suppliers
$8.00/250mg

151103-09-2
192 suppliers
$26.00/250mg
Yield: 99%
Reaction Conditions:
Stage #1:4-difluoromethoxy-3-hydroxybenzaldehyde with potassium carbonate;potassium iodide in dimethyl sulfoxide at 70; for 1 h;
Stage #2:cyclopropylcarbinyl bromide in dimethyl sulfoxide at 70; for 4 h;
Steps:
1 Example 1 : Preparation of 3-(cvclopropylmethoxy)-4-(difluoromethoxy)- benzaldehyde
55 g of 4-(difluoromethoxy)-3-hydroxybenzaldehyde, 42.42 g of K2C03 (1.05 eq), 4.86 g of Kl (0.1 eq) and 220 mL of dimethylsulphoxide (DMSO) were loaded in a reactor. The mixture was heated at 70°C and kept for 1 h. A mixture previously prepared of 42.65 g of bromomethyl cyclopropane (1.08 eq) and 1 10 mL of DMSO was added for 1 hour. The reaction was kept for 3 h at 70°C, and then cooled at room temperature. Once the temperature was reached, 375 mL of toluene was added. The suspension was filtered to remove the remaining K2C03, and then it was cooled at 0-5°C, and 375 mL of deionised water were loaded. The phases were separated and the organic phase was washed twice with 55 mL of deionised water. The solvent was removed at reduced pressure, obtaining 70 g (yield 99%) of 3-(cyclopropylmethoxy)-4-(difluoromethoxy)- benzaldehyde as a viscous yellowish fluid.
References:
INTERQUIM, S.A.;SALAET FERRE, Josep;MARQUILLAS OLONDRIZ, Francisco WO2014/60464, 2014, A1 Location in patent:Page/Page column 15
25934-52-5
55 suppliers
$52.00/250mg
1895-39-2
297 suppliers
$6.00/5g

151103-09-2
192 suppliers
$26.00/250mg
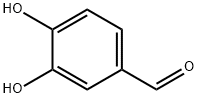
139-85-5
848 suppliers
$5.00/1g

151103-09-2
192 suppliers
$26.00/250mg
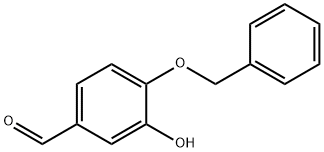
4049-39-2
69 suppliers
$80.00/1 g

151103-09-2
192 suppliers
$26.00/250mg
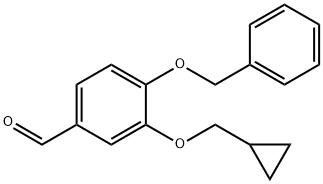
1381886-14-1
4 suppliers
inquiry

151103-09-2
192 suppliers
$26.00/250mg