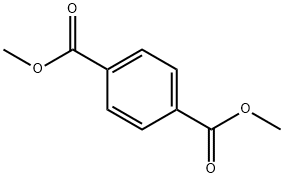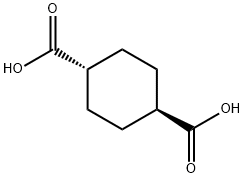
trans-1,4-Cyclohexanedicarboxybic acid synthesis
- Product Name:trans-1,4-Cyclohexanedicarboxybic acid
- CAS Number:619-82-9
- Molecular formula:C8H12O4
- Molecular Weight:172.18
100-21-0
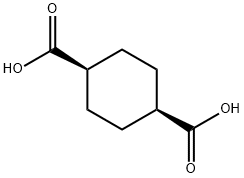
619-81-8
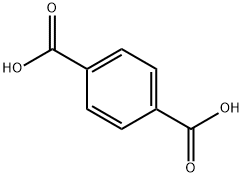
The general procedure for the synthesis of cis-1,4-cyclohexanedicarboxylic acid from terephthalic acid is as follows: 200 g of terephthalic acid, 15 g of the Pd-Pt/C catalyst described in Examples 1 to 14 and 1000 g of water were added to an autoclave and stirring was initiated. Three nitrogen purges were first performed, followed by three replacements with hydrogen. The system was pressurized to a hydrogen pressure of 4 MPa and the reaction temperature was kept stable at 200°C, and hydrogen was continuously supplied to the reaction for 3 hours. At the end of the reaction, the catalyst was removed by filtration while hot, and the filtrate was cooled and filtered again to collect the filter cake. The filter cake was dried at 120 °C to obtain the solid product. The solid product was analyzed by liquid chromatography to determine the contents of 1,4-cyclohexanedicarboxylic acid and trans-1,4-cyclohexanedicarboxylic acid therein, from which the yields of 1,4-cyclohexanedicarboxylic acid and the selectivity of trans-1,4-cyclohexanedicarboxylic acid were calculated, and the specific results are shown in Table 2.

100-21-0
591 suppliers
$6.00/25g

619-82-9
226 suppliers
$5.00/5g
Yield:619-82-9 92%
Reaction Conditions:
with 5%-palladium/activated carbon;hydrogen in water at 150; under 30003 Torr; for 5.5 h;
Steps:
1
100 parts by mass of terephthalic acid, 3.8 parts by mass of a catalyst (5% Pd / C manufactured by NE Chemcat Corporation) and 560 parts by mass of water were charged into a stainless steel reactor equipped with a stirrer, a thermometer and a gas- Thereafter, the mixture was heated to 150 DEG C under stirring at 400 rpm under atmospheric pressure.The supply of hydrogen was intermittently started so that the pressure became 4 MPa (gauge pressure) when the temperature reached 150 DEG C, and the reaction was carried out for 5.5 hours.After completion of the reaction, the reaction mixture was cooled to room temperature to obtain a reaction slurry. 3300 parts by mass of water was added to the slurry, and the product was dissolved by heating at 90 DEG C, followed by filtration to remove the catalyst.A part of the filtrate was collected and analyzed by gas chromatography. The conversion of terephthalic acid was 99% or more, the yield of 1,4-cyclohexanedicarboxylic acid was 92%, and the trans-ester ratio thereof was 36 mol%.
References:
KR101598769,2016,B1 Location in patent:Paragraph 0651 - 0655
10028-70-3
116 suppliers
$24.26/5g

619-82-9
226 suppliers
$5.00/5g

619-81-8
115 suppliers
$6.00/250mg

619-81-8
115 suppliers
$6.00/250mg

619-82-9
226 suppliers
$5.00/5g