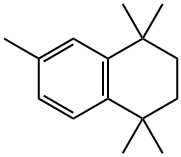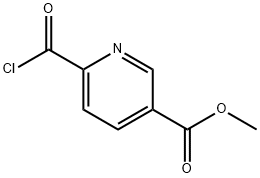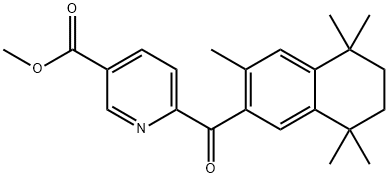
6-(3,5,5,8,8-PENTAMETHYL-5,6,7,8-TETRAHYDRO-NAPHTHALENE-2-CARBONYL)-NICOTINIC ACID METHYL ESTER synthesis
- Product Name:6-(3,5,5,8,8-PENTAMETHYL-5,6,7,8-TETRAHYDRO-NAPHTHALENE-2-CARBONYL)-NICOTINIC ACID METHYL ESTER
- CAS Number:153559-92-3
- Molecular formula:C23H27NO3
- Molecular Weight:365.47
Yield:153559-92-3 54%
Reaction Conditions:
with aluminum (III) chloride in dichloromethane at 20;Reflux;
Steps:
Methyl 6-[(3,5,5,8,8-PentamethyI-5,6,7,8-tetrahydronaphthalen-2-yl)carbonyl]nicotinate (13)
To a 2-neck, 50 mL round bottom flask equipped with a reflux condenser and magnetic stir-bar was added 12 (1.40 g, 6.91 mmol) followed by a solution of crude acid chloride 11 (6.62 mmol) in DCM (15 mL). Aluminum chloride (2.20 g, 16.5 mmol) was added to the reaction solution at room temperature slowly, with stirring, and the reaction solution turned from colorless to red accompanied by the evolution of gas and heat. The reaction was stirred for 5 min then heated to reflux for 15 min. The reaction was judged to be complete by TLC, and the solution was poured into an ice solution (25 mL) acidified with a 20% HCl solution (8 mL) and ethyl acetate was added (13 mL). The aqueous and organic layers were separated, and the aqueous layer was extracted with ethyl acetate (15 mL, twice). The combined organics were washed with water and brine, dried over sodium sulfate, filtered and concentrated to give crude 13. Crude 13 was purified by column chromatography (250 mL Si02, hexanes:ethyl acetate 95:5 to 85:15) to give 13 (1.30 g, 54%) as an off-white, crystalline solid: ? NMR (400 MHz, CDCl3) ? 9.26 (dd, J= 2.0, 0.8, 1H), 8.49 (dd, J= 8.0, 2.0, 1H), 8.10 (dd, J= 8.0, 0.8, 1H), 7.42 (s, 1H), 7.21 (s, 1H), 3.99 (s, 3H), 2.39 (s, 3H), 1.68 (s, 4H), 1.29 (s, 6H), 1.20 (s, 6H); 13C NMR (100.6 MHz, CDCl3) ? 195.8, 165.1 , 158.6, 149.9, 149.2, 141.6, 138.1, 135.7, 133.2, 130.3, 129.7, 127.5, 123.8, 52.7, 34.8, 34.8, 34.4, 33.8, 31.6, 31.5, 20.5; LC-MS (M+H)+ calcd for C23H28N03 366.2069, found 366.2070.
References:
WO2013/40227,2013,A2 Location in patent:Page/Page column 21; 22
17874-79-2
132 suppliers
$15.00/1g

153559-92-3
10 suppliers
inquiry
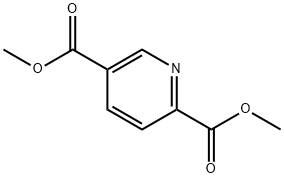
881-86-7
177 suppliers
$28.00/1g

153559-92-3
10 suppliers
inquiry
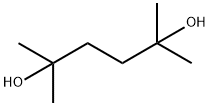
110-03-2
360 suppliers
$6.00/25g

153559-92-3
10 suppliers
inquiry
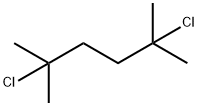
6223-78-5
134 suppliers
$9.00/5g

153559-92-3
10 suppliers
inquiry