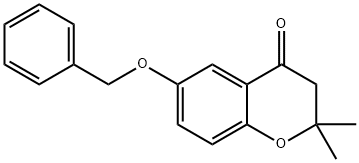
4H-1-Benzopyran-4-one, 2,3-dihydro-2,2-dimethyl-6-(phenylmethoxy)- synthesis
- Product Name:4H-1-Benzopyran-4-one, 2,3-dihydro-2,2-dimethyl-6-(phenylmethoxy)-
- CAS Number:171876-69-0
- Molecular formula:C18H18O3
- Molecular Weight:282.33
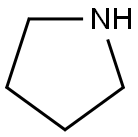
123-75-1
574 suppliers
$10.00/5g
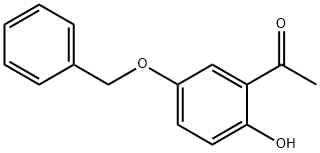
30992-63-3
77 suppliers
$45.00/250mg

171876-69-0
1 suppliers
inquiry
Yield:171876-69-0 13.6 g (47%)
Reaction Conditions:
in acetone;toluene;
Steps:
100 6-Benzyloxy-2,2-dimethylchroman-4-one
EXAMPLE 100 6-Benzyloxy-2,2-dimethylchroman-4-one A mixture of 25.0 g (0.103 mole) 2-hydroxy-5-benzyloxyacetophenone, 8 mL (6.0 g, 0.130 mole) acetone and 4 mL (3.7 g, 0.052 mole) pyrrolidine in 100 mL toluene was stirred at room temperature for 4 hours, then refluxed over a Dean-Stark trap for 3 hours. An additional 8 mL (6.0 g, 0.130 mole) acetone and 4 mL (3.7 g, 0.052 mole) pyrrolidine was added and the mixture refluxed for 18 hours. The mixture was concentrated, poured into water, basified to pH 8 with ammonium hydroxide, then extracted with ether. The ether extract was washed successively with 1N KOH, 1N HCl, water and brine, then dried (MgSO4) and concentrated to give an oil. Flash chromatography on silica gel eluding with 20% ethyl acetate in hexanes afforded a solid. Recrystallization from ethyl acetate in hexanes afforded a solid. Recrystallization from ethyl acetate in hexanes yielded the title product as a solid 13.6 g (47%), mp 105°-107° C. NMR (CDCl3): δ1.4 (s, 6H); 2.7 (s, 2H); 5.1 (s, 2H). Analysis calculated for C18 H18 O3: C, 76.57; H, 6.43. Found: C, 76.16; H, 6.42.
References:
US5641789,1997,A
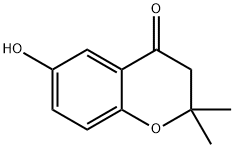
31366-85-5
51 suppliers
$45.00/25mg

100-39-0
430 suppliers
$10.00/10g
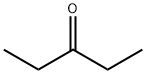
96-22-0
297 suppliers
$9.00/1g

171876-69-0
1 suppliers
inquiry

30992-63-3
77 suppliers
$45.00/250mg

67-64-1
0 suppliers
$19.10/10ml

171876-69-0
1 suppliers
inquiry
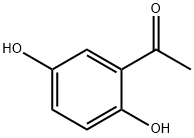
490-78-8
358 suppliers
$5.00/50mg

171876-69-0
1 suppliers
inquiry