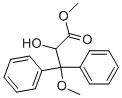
Benzenepropanoic acid,a-hydroxy-b-methoxy-b-phenyl-,methyl ester synthesis
- Product Name:Benzenepropanoic acid,a-hydroxy-b-methoxy-b-phenyl-,methyl ester
- CAS Number:178306-47-3
- Molecular formula:C17H18O4
- Molecular Weight:286.32

67-56-1
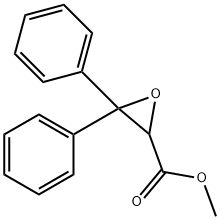
76527-25-8

178306-47-3
1. 500 g (2.74 mol) of benzophenone was dissolved in 1200 mL of toluene and the reaction system was purged using nitrogen. Subsequently, 267 g (4.94 mol) of sodium methanol was added and stirred to form a suspension. 2. The suspension was cooled and 410 mL (4.68 mol) of methyl chloroacetate in toluene (300 mL) was slowly added dropwise over a period of 90 minutes, with the reaction temperature maintained at -8 to 4°C. The reaction temperature was maintained at -8 to 4°C. After the dropwise addition was completed, the reaction solution was cooled to 0°C and maintained for 1 hr. 3. 1000 mL of water was added and stirred for 15 minutes before liquid-liquid separation. The organic layer was washed with 1000mL of water. 4. The solvent was removed by distillation under reduced pressure and the resulting residue (methyl (RS)-3,3-diphenyl-2,3-epoxyacrylate) was dissolved in 1200 mL of methanol and cooled to 0°C. 5. A methanolic solution of 15 g (0.08 mol) of toluene sulfonic acid monohydrate (150 mL) was added slowly and dropwise over 30 min. The mixture was stirred at 0°C for 1.5 h. The precipitated crystals were collected by filtration. 6. The crystals were dissolved in 2500 mL of ethyl acetate and washed twice with 1000 mL of 5% aqueous sodium carbonate. 7. The solvent was removed by pressurized distillation and 1000 mL of hexane was added to the residue, suspended and stirred at room temperature for 1.5 hours, followed by stirring at 0°C for 1 hour. 8. The crystals were collected by filtration and dried under reduced pressure at 60 °C for 5 h to give 659 g (yield: 84%) of methyl (RS)-2-hydroxy-3-methoxy-3,3-diphenylpropionate.

67-56-1
790 suppliers
$7.29/5ml-f

76527-25-8
54 suppliers
$140.00/100mg

178306-47-3
152 suppliers
$60.00/500mg
Yield:178306-47-3 88%
Reaction Conditions:
with boron trifluoride diethyl etherate at 0 - 20; for 14 h;
Steps:
1 Methyl 2-hydroxy-3-methoxy-3,3-diphenylpropionate
5 g (19.6 mmol) of methyl 3,3-diphenyl-2,3-epoxypropionate,Was dissolved in 50 ml of anhydrous methanol,0.1 ml of boron trifluoride etherate was added at 0 ° C.The mixture was stirred at 0 ° C. for 2 hours,The mixture was further stirred at room temperature for 12 hours.The solvent was distilled off, The residue was placed in ethyl acetate,Washed with sodium bicarbonate and water,And dried over magnesium sulfate.After removing the solvent by distillation,5.5 g (88%) of a pale yellow oil remained.
References:
ABBVIE DEUTSCHLAND GMBH & CO. KG;RIECHERS, HARTMUT;KLINGE, DAGMAR;AMBERG, WILHELM;KLING, ANDREAS;MULLER, STEFAN;BAUMANN, ERNST;RHEINHEIMER, JOACHIM;VOGELBACHER, UWE JOSEF;WERNET, WOLFGANG;UNGER, LILIANE;RASCHACK, MANFRED JP5700378, 2015, B2 Location in patent:Paragraph 0062

76527-25-8
54 suppliers
$140.00/100mg

178306-47-3
152 suppliers
$60.00/500mg
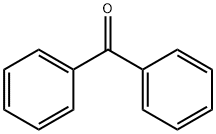
119-61-9
820 suppliers
$5.00/10g
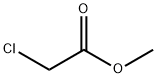
96-34-4
364 suppliers
$10.00/25g

178306-47-3
152 suppliers
$60.00/500mg
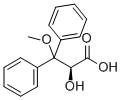
178306-52-0
196 suppliers
$106.00/100mg

178306-47-3
152 suppliers
$60.00/500mg

119-61-9
820 suppliers
$5.00/10g

178306-47-3
152 suppliers
$60.00/500mg