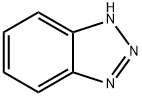
1H-Benzotriazole synthesis
- Product Name:1H-Benzotriazole
- CAS Number:95-14-7
- Molecular formula:C6H5N3
- Molecular Weight:119.12
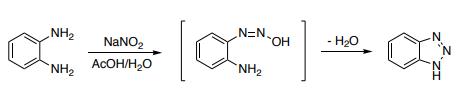
Synthesis of 1H-benzotriazole via diazotization of o-phenylenediamine
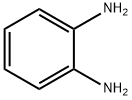
Reaction: Add o-phenylenediamine to 50°C water to dissolve, then add glacial acetic acid, cool down to 5°C, add sodium nitrite to stir the reaction. The reactant gradually turned dark green, the temperature rose to 70-80 ℃, the solution turned orange-red, placed at room temperature for 2 hours, cooled, filtered out the crystals, washed with ice water, dried to obtain the crude product, the crude product was distilled under reduced pressure, and collected 201 -204°C (2.0kPa) fraction, and then recrystallized with benzene to obtain 1H-Benzotriazole products with a melting point of 96-97°C, with a yield of about 80%.
95-54-5
541 suppliers
$9.00/1g

95-14-7
919 suppliers
$6.00/100g
Yield:95-14-7 99.9%
Reaction Conditions:
with sulfuric acid;NaNO2 in methanol;
Steps:
1 Benzotriazole (for comparison)
EXAMPLE 1 Benzotriazole (for comparison) 108 g of freshly distilled 99.8% pure o-phenylenediamine were suspended in 125 ml of methanol and heated to 50° C. At this temperature, gaseous methyl nitrite, prepared from sodium nitrite solution (25% strength), methanol and sulphuric acid (48% strength), was introduced. The rate of addition was such that no off-gas left the reaction flask. As soon as the reaction mixture did not absorb any more gas (about 2 hours), a sample was removed and tested for complete conversion. This was followed by first distilling off methanol and then water from the reaction mixture. With the addition of polywax (polydiol 1550) as a lubricant, the mixture was then distilled continuously through a thin-film evaporator at 4 mbar and a heater temperature of 220° C. Benzotriazole came over at about 165° C. and 4 mbar. Such freshly distilled benzotriazole is 99.9% pure, as analyzed by gas chromatography. It has a slightly yellow colour. On standing for several weeks, the pale yellow colour fades to a palate beige colour.
References:
US4918195,1990,A
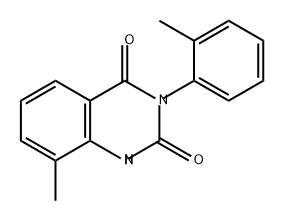
857203-66-8
0 suppliers
inquiry

95-14-7
919 suppliers
$6.00/100g
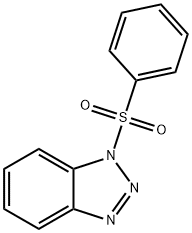
4106-18-7
13 suppliers
$264.00/1g

95-14-7
919 suppliers
$6.00/100g