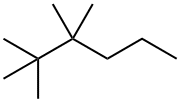
2,2,3,3-tetramethylhexane synthesis
- Product Name:2,2,3,3-tetramethylhexane
- CAS Number:13475-81-5
- Molecular formula:C10H22
- Molecular Weight:142.28
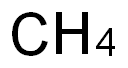
34557-54-5
0 suppliers
inquiry

562-49-2
43 suppliers
$43.10/1g
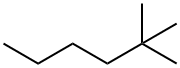
590-73-8
64 suppliers
$45.00/50mg
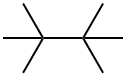
594-82-1
50 suppliers
$60.00/50mg
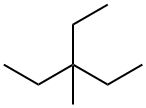
1067-08-9
32 suppliers
$186.00/5mL
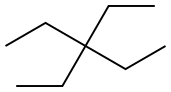
1067-20-5
13 suppliers
$906.98/1ML

13475-81-5
5 suppliers
inquiry
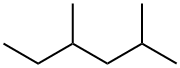
589-43-5
65 suppliers
$46.00/5mL

74-84-0
67 suppliers
$269.00/110 g

74-98-6
129 suppliers
$20.00/1ea
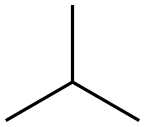
75-28-5
132 suppliers
$40.00/1g
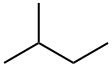
463-82-1
55 suppliers
inquiry
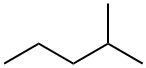
78-78-4
281 suppliers
$20.00/25ML
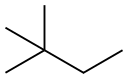
107-83-5
244 suppliers
$10.00/1g
75-83-2
129 suppliers
$35.00/1ml
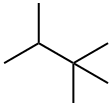
464-06-2
96 suppliers
$23.50/1g

590-35-2
75 suppliers
$34.00/1mL
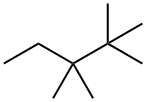
7154-79-2
9 suppliers
inquiry

1333-74-0
116 suppliers
$190.00/56L
Yield: 3.44% , 0.17% , 6.34% , 4.51% , 15.7% , 4.01% , 6.41% , 0.4% , 1.2% , 3.52% , 1.89% , 1.45% , 9.67% , 1.45% , 1.72% , 9.66% , 2.18% , 5.82%
Reaction Conditions:
with water at 84;Product distribution / selectivity;Photolysis;
Steps:
1 EXAMPLE 1
Example 1 Photochemical Studies [0027] This example demonstrates the process of the present invention in which the energy source used to generate hydrated electrons is ultra violet radiation. [0028] The use of ultra violet radiation in the process of the present invention is described in detail in U.S. Provisional Application Serial No. 60/366,068 filed Mar. 19, 2002, which is incorporated herein in its entirety by this reference. [0029] Continuous-flow photolysis studies were conducted in a 5.7-liter, 4-inch-diameter, 28-inch-long cylindrical stainless steel reactor. The apparatus was designed to accommodate a 32-inch long low-pressure mercury vapor lamp. The reactor was fitted with a 100-Watt Sunlight Systems UV lamp with GE-214 quartz envelope to provide 85% transmittance of 185-nm photon emission. [0030] For the initial test, the reactor was charged with approximately 24 psig ultra-pure methane (99.7%) and heated to 84° C. Upon reaching the target temperature, the ultraviolet light source was energized and methane control valve opened to achieve a methane flow rate of 36 mls/min. Samples of process gas were collected through a septum in the reactor exhaust line and analyzed for hydrogen and light hydrocarbon content (H2, ethane, ethylene, acetylene, CO2 and CO) with a dual-channel MicroGC gas chromatography (GC) using Molecular Sieve 5A PLOT and PoraPLOT U columns. Higher molecular weight products were characterized by a chromatograph/mass spectrometer (GC/MS) with a Restek Rtx-1 column. [0031] In the second study, the reactor was charged with approximately 250 mls deoxygenated deionized water and flushed with methane. The system was heated to 84° C. and additional methane added to a system pressure of 24 psig. Ultra-pure methane was processed through the photoreactor at a flow rate of 34 ml/min. The results from the photochemical studies are summarized in the following table
References:
Sherwood, Steven P. US2003/233019, 2003, A1 Location in patent:Page column 3-4
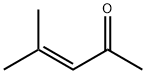
141-79-7
283 suppliers
$10.00/5g

13475-81-5
5 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

13475-81-5
5 suppliers
inquiry

65995-72-4
0 suppliers
inquiry

13475-81-5
5 suppliers
inquiry