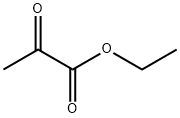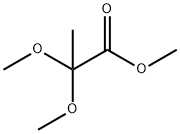
2,2-DIMETHOXYPROPIONIC ACID METHYL ESTER synthesis
- Product Name:2,2-DIMETHOXYPROPIONIC ACID METHYL ESTER
- CAS Number:10076-48-9
- Molecular formula:C6H12O4
- Molecular Weight:148.16
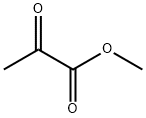
600-22-6
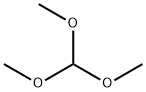
149-73-5

10076-48-9
General procedure for the synthesis of methyl 2,2-dimethoxypropionate from methyl pyruvate and trimethyl orthoformate: 100 g (979 mmol) of methyl pyruvate was mixed with 135 g (1273 mmol) of trimethyl orthoformate in 240 ml of methanol. After adding 0.96 g (9.79 mmol) of concentrated sulfuric acid, the mixture was heated to reflux for 4 hours. Subsequently, the solvent was distilled out over 2 h. The crude product was cooled to 10 °C and added to a solution of 2.4 g of potassium hydroxide in 1200 ml of water at 10 °C. After repeated extraction with ether, the organic phases were combined and dried over anhydrous sodium sulfate, filtered and concentrated. The residue was distilled under reduced pressure and the fractions with boiling points of 50-55°C (10 mbar) were collected. Finally 118 g (77% yield) of the target product was obtained.1H NMR (CDCl3) data were as follows: δ = 1.53 (s, 3H, C-CH3), 3.29 (s, 6H, CH3-OCO-CH3), 3.82 (s, 3H, COOCH3).

67-56-1
790 suppliers
$7.29/5ml-f

600-22-6
392 suppliers
$6.00/5g

149-73-5
548 suppliers
$12.00/5g

10076-48-9
61 suppliers
$17.00/1g
Yield: 62%
Reaction Conditions:
with sulfuric acid for 4 h;Reflux;
Steps:
1.1
Svnthesis of S-Methoxy-pyrrolidine-S-carboxylic acid methyl ester Step 1 : Preparation of methyl α, α-dimethoxypropionate The procedure by Ernest Wenkert, et al. (JACS, 1983, 705, 2021 -2029) was followed. A solution of methyl pyruvate (44g), trimethyl orthoformate (62 ml), concentrated H2SO4 (0.2 ml) in MeOH (120 ml) was reluxed for 4 hours. In the next one hour period, solvent (about 80 ml) was distilled out. The reaction mixture was cooled to 10 0C, poured into a KOH solution (1 .2 g KOH in 600 ml water), and extracted with ether (3x). Combined ether extracts were washed with brine and dried (MgSO4). After concentration, the residue was distilled under vacuum to provide the acetal (8BH) (4Og, 62%, 40-43C/1 torr).
References:
SCHERING CORPORATION WO2009/105500, 2009, A1 Location in patent:Page/Page column 215

600-22-6
392 suppliers
$6.00/5g

149-73-5
548 suppliers
$12.00/5g
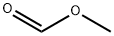
107-31-3
355 suppliers
$16.00/25mL

10076-48-9
61 suppliers
$17.00/1g

67-56-1
790 suppliers
$7.29/5ml-f
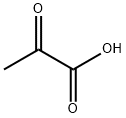
127-17-3
568 suppliers
$5.00/10g

10076-48-9
61 suppliers
$17.00/1g