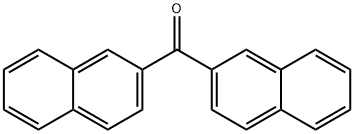
2,2'-DINAPHTHYL KETONE synthesis
- Product Name:2,2'-DINAPHTHYL KETONE
- CAS Number:613-56-9
- Molecular formula:C21H14O
- Molecular Weight:282.34
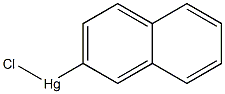
4809-95-4

613-56-9
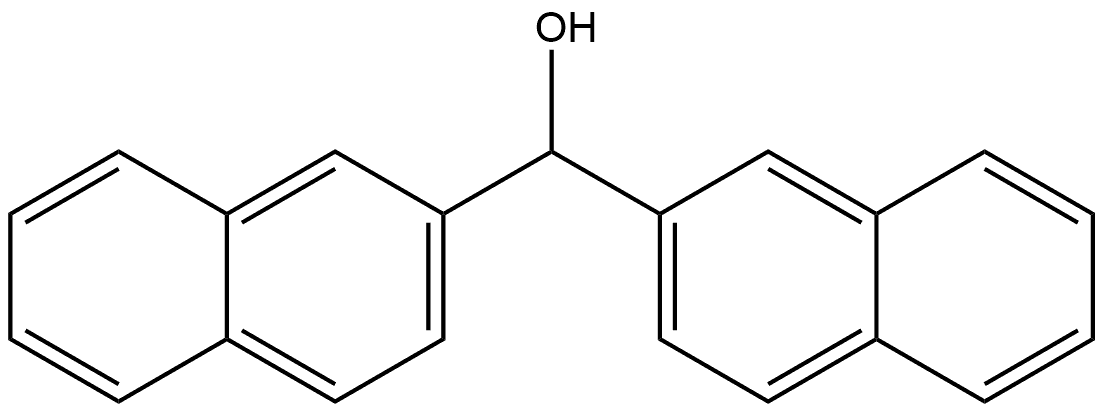
General procedure for the synthesis of 2,2'-naphthalenyl ketones from di(naphthalen-2-yl)methanol: According to the modified method of Azuma et al. (Tetrahedron, 2013, 69(6), 1694-1699), MnO2 (8.4 g, 96.7 mmol, 10 eq.) was added to stirred solution of di(naphthalen-2-yl)methanol (2.75 g 9.68 mmol, 1.0 equiv) in a solution of dichloromethane (DCM, 20 mL). The reaction was carried out at room temperature with continuous stirring for 24 hours. Upon completion of the reaction, the mixture was filtered through Celite and the filtrate was concentrated under reduced pressure to afford the target product 2,2'-naphthyl ketone (2.70 g, 9.56 mmol, 99% yield) as a colorless solid. The product could be used in the subsequent reaction without further purification. 2H); 13C NMR (100 MHz, CDCl3) δC/ppm 196.8 (1C), 135.3 (2C), 135.2 (2C), 132.3 (2C), 131.8 (2C), 127.8 (4C), 126.8 (4C), 125.9 (4C); MS (ESI) [M + Na]+: 305.1 (100); IR (vmax/cm-1): 2915, 1653, 1379, 1334, 1194, 763.
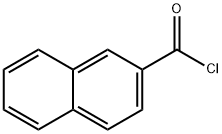
201230-82-2
1 suppliers
inquiry
32316-92-0
476 suppliers
$6.00/1g

613-56-9
35 suppliers
$36.00/100mg
Yield: 65%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);1,3-bis-(diphenylphosphino)propane;silver nitrate in acetone at 40; under 760.051 Torr; for 24 h;Autoclave;Inert atmosphere;
Steps:
General Procedure for the Carbonylation of ArylboronicAcids
General procedure: The reaction was carried out in an autoclave containing a 10mL Teflon reaction tube. Pd(PPh3)4 (0.02 mmol), DPPP(0.04 mmol), and a magnetic stir bar were placed in the tubewhich was then capped with a stopper and flushed withargon. Then, aryl boronic acid (1 mmol), AgNO3 (1 mmol),and acetone (3 mL) were added to the tube. The tube was putinto the autoclave. Once sealed, the autoclave was purgedseveral times with CO, pressurized to 1 atm at r.t. and thenheated in an oil bath at 40 °C for 24 h. The autoclave wasthen cooled to r.t. and carefully vented to discharge CO in afume hood. Water (10 mL) was added, and the product wasextracted with CH2Cl2 (3 × 15 mL). The organic layers werewashed with brine, dried over Na2SO4, and evaporated. Thecrude product was purified by column chromatography onsilica gel using a mixture of EtOAc and PE as eluent to givethe products. The identity and purity of the product wasconfirmed by 1H NMR and 13C NMR spectroscopy and MSor HRMS spectrometry.
References:
Li, Yang;Lu, Wei;Xue, Dong;Wang, Chao;Liu, Zhao-Tie;Xiao, Jianliang [Synlett,2014,vol. 25,# 8,art. no. ST-2014-W0057-L,p. 1097 - 1100] Location in patent:supporting information

4809-95-4
0 suppliers
inquiry

613-56-9
35 suppliers
$36.00/100mg