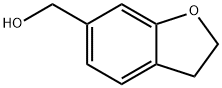
(2,3-dihydrobenzofuran-6-yl)methanol synthesis
- Product Name:(2,3-dihydrobenzofuran-6-yl)methanol
- CAS Number:1083168-69-7
- Molecular formula:C9H10O2
- Molecular Weight:150.17
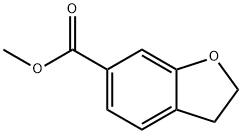
1083168-68-6

1083168-69-7
Procedure: synthesis of (2,3-dihydrobenzofuran-6-yl)methanol; A THF solution of methyl 2,3-dihydrobenzofuran-6-carboxylate (17.8 g, 100 mmol) was slowly added to a stirred tetrahydrofuran (THF, 300 mL) solution of lithium aluminium hydride (6.1 g, 250 mmol) at 0 °C. The reaction mixture was continued to be stirred at room temperature for 1 hour. Upon completion of the reaction, the reaction was quenched by careful addition of saturated aqueous sodium hydroxide solution, followed by extraction of the mixture with ethyl acetate. The organic layers were combined, washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to afford (2,3-dihydrobenzofuran-6-yl)methanol (13.8 g, 92.0% yield). The product was characterized by 1H NMR (400 MHz, CDCl3): δ 7.17 (d, J = 7.2 Hz, 1H), 6.84 (d, J = 7.2 Hz, 1H), 6.81 (s, 1H), 4.62 (s, 2H), 4.58 (t, J = 8.4 Hz, 2H), 3.20 (t, J = 8.4 Hz, 2H), 1.67 ( br s, 1H).

1083168-68-6
18 suppliers
inquiry

1083168-69-7
54 suppliers
$45.00/10mg
Yield:1083168-69-7 92%
Reaction Conditions:
Stage #1: methyl 2,3-dihydrobenzofuran-6-carboxylatewith lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 1 h;
Stage #2: with sodium hydroxide;water in tetrahydrofuran;
Steps:
G.f
Stepf: (2,3-Dihydrobenzofuran-6-yl)methanol; To a stirred solution of lithium aluminium hydride (6.1 g, 250 mmol) in THF(300 mL) was added a solution of methyl 2,3-dihydrobenzofuran-6-carboxylate (17.8 g, 100 mmol) in THF at 0°C. The mixture was stirred at room temperature for 1 h. A saturated aqueous NaOH solution was added and the mixture was extracted with ethyl acetate. The combined organic layers were washed with brine and dried over Na2SO4, filtered and concentrated to afford (2,3-dihydrobenzofuran-6-yl)methanol (13.8 g, 92.0% yield). 1H NMR (400 MHz, CDCl3) δ: 7.17 (d, J= 7.2, IH), 6.84 (d, J= 7.2, IH), 6.81 (s, IH), 4.62 (s, 2H), 4.58 (t, J= 8.4, 2H), 3.20 (t, J= 8.4, 2H),) 1.67 (br s, IH).
References:
WO2008/141119,2008,A2 Location in patent:Page/Page column 77; 79

50-00-0
898 suppliers
$10.00/25g
189035-22-1
81 suppliers
$65.00/50mg

1083168-69-7
54 suppliers
$45.00/10mg
13196-11-7
112 suppliers
$185.00/250mg

1083168-69-7
54 suppliers
$45.00/10mg
![6-Hydroxy-2,3-dihydrobenzo[b]furan-3-one](/CAS/GIF/6272-26-0.gif)
6272-26-0
201 suppliers
$9.00/1g

1083168-69-7
54 suppliers
$45.00/10mg
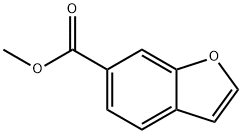
588703-29-1
72 suppliers
$122.00/100mg

1083168-69-7
54 suppliers
$45.00/10mg