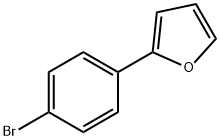
2-(4-BROMOPHENYL)FURAN synthesis
- Product Name:2-(4-BROMOPHENYL)FURAN
- CAS Number:14297-34-8
- Molecular formula:C10H7BrO
- Molecular Weight:223.07
13331-23-2
346 suppliers
$13.43/250mgs:
589-87-7
540 suppliers
$10.00/1g

14297-34-8
33 suppliers
$68.00/250mg
Yield:14297-34-8 94%
Reaction Conditions:
with tetrabutylammomium bromide;sodium carbonate;triphenylphosphine;palladium 10% on activated carbon in tetrahydrofuran;water at 60;
Steps:
A.1
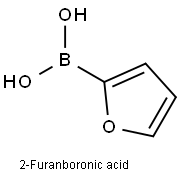
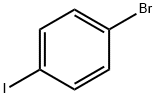
Preparation of intermediate 1 A lO L vessel under N2 was charged with 4-bromoiodobenzene (301 g; 1,06 moles), 2-furanboronic acid (148.81 g; 1,25 moles), triphenylphosphine (13.95 g; 0.05 moles), tetra-n-butylammonium bromide (377.29 g; 1.1 moles), sodium carbonate (225.53 g; 2 moles), palladium on carbon 10% (60.20 g; 56.57 mmoles), tetrahydrofuran (3.72 L) and water (3.72 L). The reaction mixture was heated at 60 0C for 20 until gas chromatography showed no unreacted starting material. After cooling to room temperature, the reaction mixture was filtered over decalite. The organic layer was separated and washed with a saturated NaCl solution (2 L), concentrated under reduced pressure and diluted with toluene (1.5 L). The solids which formed were filtered off and washed with toluene (1 L). The combined toluene layers were concentrated and purified by column chromatography (silica gel; hexane). The product was crystallized from ethano I/water (2.27 L / 2.27 L) and dried. Yield: 266 g of intermediate 1 (2-(4- bromophenyl)-furan) (94%).
References:
WO2008/40669,2008,A2 Location in patent:Page/Page column 7

13331-23-2
346 suppliers
$13.43/250mgs:
106-37-6
479 suppliers
$9.00/5g

14297-34-8
33 suppliers
$68.00/250mg
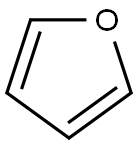
110-00-9
218 suppliers
$10.00/25g
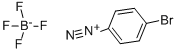
673-40-5
121 suppliers
$52.00/5g

14297-34-8
33 suppliers
$68.00/250mg
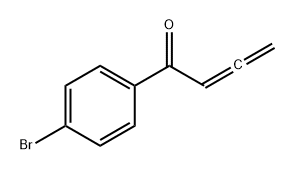
378186-96-0
3 suppliers
inquiry

14297-34-8
33 suppliers
$68.00/250mg

110-00-9
218 suppliers
$10.00/25g

106-40-1
483 suppliers
$12.00/5g

14297-34-8
33 suppliers
$68.00/250mg