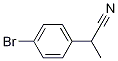
2-(4-broMophenyl)propanenitrile synthesis
- Product Name:2-(4-broMophenyl)propanenitrile
- CAS Number:42186-06-1
- Molecular formula:C9H8BrN
- Molecular Weight:210.07
16532-79-9

74-88-4

42186-06-1
Example 649 Synthesis of 2-(4-bromophenyl)propionitrile: 2-(4-bromophenyl)acetonitrile (5.0 g, 25.5 mmol) was dissolved in anhydrous THF (70 mL) and cooled to 0°C. Sodium hydride (60 wt%, 1.5 g, 38.3 mmol) was added batchwise under stirring. The reaction mixture was stirred at room temperature for 1 hour. Subsequently, methyl iodide (1.8 mL, 28.1 mmol) was added and stirring was continued for 14 hours. Upon completion of the reaction, the reaction was carefully quenched with water at 0 °C and diluted with ethyl acetate (200 mL). The organic layer was separated, dried over anhydrous sodium sulfate, filtered and concentrated. The residue was purified by column chromatography (silica gel, 0-30% ethyl acetate/heptane gradient elution) to afford the target product, 2-(4-bromophenyl)propionitrile (3.8 g, 72%), as a yellow oil.ESI MS m/z 210 [C9H8BrN + H]+.
Yield:42186-06-1 91%
Reaction Conditions:
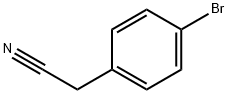
Stage #1: 4-Bromophenylacetonitrile in tetrahydrofuran at -78; for 0.5 h;Inert atmosphere;
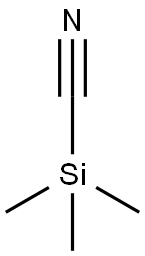
Stage #2: methyl iodide in tetrahydrofuran; for 1 h;Inert atmosphere;
References:
Turnbull, Ben W. H.;Evans, P. Andrew [Journal of the American Chemical Society,2015,vol. 137,# 19,p. 6156 - 6159] Location in patent:supporting information
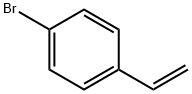
2039-82-9
306 suppliers
$10.00/1g
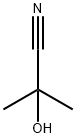
75-86-5
0 suppliers
$64.90/5G

42186-06-1
50 suppliers
inquiry
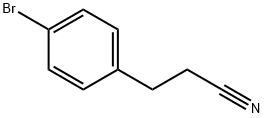
57775-08-3
88 suppliers
$45.00/50mg