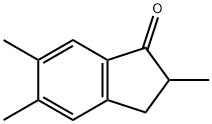
2,5,6-TriMethyl-2,3-dihydro-1H-inden-1-one synthesis
- Product Name:2,5,6-TriMethyl-2,3-dihydro-1H-inden-1-one
- CAS Number:89044-51-9
- Molecular formula:C12H14O
- Molecular Weight:174.24
Yield:89044-51-9 21%
Reaction Conditions:
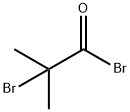
Stage #1: 2-bromoisobutyric acid bromidewith aluminum (III) chloride in dichloromethane at 0; for 1 h;
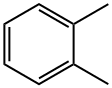
Stage #2: o-xylene in dichloromethane at 20;
Steps:
9 2,5,6-Trimethylindan-1-one
Example 9
2,5,6-Trimethylindan-1-one
To a suspension of 557 g (4.2 mol) AlCl3 in 500 ml of CH2Cl2 362 g (1.58 mol) of 2-bromo-2-methylpropanoyl bromide was added dropwise with vigorous stirring over 15 min at 0° C.
This mixture was stirred for 45 min at this temperature; then, a solution of 167 g (1.58 mol) of o-xylene in 200 ml of CH2Cl2 was added dropwise.
The mixture was slowly warmed to ambient temperature, stirred additionally overnight, and then poured onto 2000 cm3 of ice.
The organic layer was separated, and the aqueous layer was extracted with 3*500 ml of CH2Cl2.
The combined extract was dried over MgSO4 and evaporated to dryness.
Fractional distillation gave a yellowish mixture of the title indanones, b.p. 143-148° C./7 mm Hg.
This mixture was recrystallized from 800 ml of n-hexane.
Crystals of 2,5,6-trimethylindan-1-one precipitated at -30° C. were filtered off, washed with 2*40 ml of cold n-hexane, and dried in vacuum.
Yield 57.8 g (21%) of 2,5,6-trimethylindan-1-one.
Anal. calc. for C12H14O: C, 82.72; H, 8.10. Found: C, 82.74; H, 8.12.
1H NMR (CDCl3): δ 7.49 (s, 1H, 4-H), 7.09 (s, 1H, 7-H), 3.29 (dd, J=16.9 Hz, J=7.6 Hz, 1H, CHMe), 2.67-2.59 (m, 2H, CH2), 2.32 (s, 3H, Me), 2.28 (s, 3H, Me), 1.27 (d, J=7.6 Hz, MeCH).
References:
US8569532,2013,B2 Location in patent:Page/Page column 65
20769-85-1
265 suppliers
$13.00/5g
95-47-6
616 suppliers
$10.00/25g

89044-52-0
0 suppliers
inquiry

89044-51-9
8 suppliers
inquiry

50-00-0
892 suppliers
$10.00/25g
![Propanedioic acid, methyl[(3-methylphenyl)methyl]-, diethyl ester (9CI)](/CAS/20210305/GIF/94430-87-2.gif)
94430-87-2
0 suppliers
inquiry
89044-50-8
21 suppliers
inquiry

89044-52-0
0 suppliers
inquiry

89044-51-9
8 suppliers
inquiry