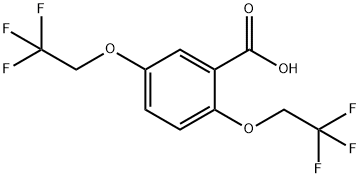
2,5-Bis(2,2,2-trifluoroethoxy)benzoic acid synthesis
- Product Name:2,5-Bis(2,2,2-trifluoroethoxy)benzoic acid
- CAS Number:35480-52-5
- Molecular formula:C11H8F6O4
- Molecular Weight:318.17
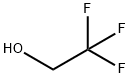
75-89-8
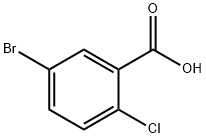
21739-92-4

35480-52-5
Example 1: At room temperature, 300 mL of tetrahydrofuran (THF) was added to a reaction vial and 84.7 g of potassium tert-butanolate was added with stirring. Subsequently, 76.0 g of 2,2,2-trifluoroethanol was slowly added dropwise to the reaction mixture, controlling the reaction temperature to not exceed 35 °C. After the dropwise addition, the reaction mixture was continued to be stirred and then 29.6 g of 5-bromo-2-chlorobenzoic acid was added. Next, 27.3 g of copper (I) bromide was added and the reaction mixture was heated to reflux. After 43 hours of reaction, the mixture was cooled to 5 °C and slowly poured into dilute hydrochloric acid. The organic and aqueous phases were separated. The solvent was removed by distillation, during which the product gradually precipitated. 100 mL of water was added to the residue and the solid product was collected by filtration. To purify the product, the crude product was dissolved in methyl tertiary butyl ether (MTB ether), filtered through a neutral alumina column to remove insoluble components, and subsequently evaporated to remove the solvent. Finally, 2,5-bis(2,2,2-trifluoroethoxy)benzoic acid was recrystallized by solvent recrystallization in ethanol/water mixture to give 2,5-bis(2,2,2-trifluoroethoxy)benzoic acid in 45% yield. The product had a melting point of 120-122°C and was analyzed by high performance liquid chromatography (HPLC) with a purity greater than 98%.

75-89-8
488 suppliers
$9.00/1g

21739-92-4
693 suppliers
$8.00/10g

35480-52-5
272 suppliers
$13.00/1g
Yield:35480-52-5 93.5%
Reaction Conditions:
with C10H12N2O8(4-)*Cu(2+)*2H4N(1+) in N,N-dimethyl-formamide at 20; for 4 h;Reagent/catalyst;
Steps:
1-23; 1-4
S1,After mixing 5mol of EDTA-copper ammonium complex with 5mol of DMF solvent,Put 1mol of 5-bromo-2-chlorobenzoic acid and trifluoroethanol and mix well,React at 20°C for 4h;
S2,The reaction system in step S1 is diluted with 5ml of water,Then adjust the pH to 6 to precipitate crystals;
S3. The crystals precipitated in the step S2 of separation are 2,5-bis(2,2,2-trifluoroethoxy)benzoic acid.
References:
CN112010748,2020,A Location in patent:Paragraph 0026-0043; 0045

1023951-32-7
0 suppliers
inquiry

35480-52-5
272 suppliers
$13.00/1g
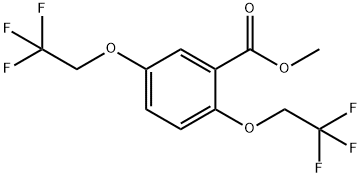
35480-31-0
54 suppliers
$19.00/1g

35480-52-5
272 suppliers
$13.00/1g
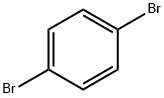
106-37-6
477 suppliers
$9.00/5g

35480-52-5
272 suppliers
$13.00/1g
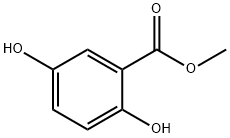
2150-46-1
243 suppliers
$6.00/1g

35480-52-5
272 suppliers
$13.00/1g