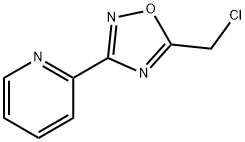
2-[5-(Chloromethyl)-1,2,4-oxadiazol-3-yl]pyridine synthesis
- Product Name:2-[5-(Chloromethyl)-1,2,4-oxadiazol-3-yl]pyridine
- CAS Number:90002-06-5
- Molecular formula:C8H6ClN3O
- Molecular Weight:195.61
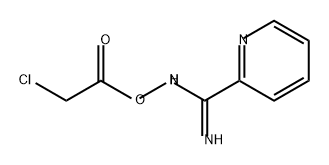
749932-24-9
0 suppliers
inquiry
![2-[5-(Chloromethyl)-1,2,4-oxadiazol-3-yl]pyridine](/CAS/GIF/90002-06-5.gif)
90002-06-5
39 suppliers
$63.00/50mg
Yield:90002-06-5 96%
Reaction Conditions:
in toluene; for 2 h;Reflux;Molecular sieve;
Steps:
239.3
Step-3: The step-2 product (18 g, 85 mmol) was refluxed in toluene (200 ml) along with 4 molecular sieves for 2 hrs. The reaction mixture was concentrated under vacuum to provide a crude residue. The crude residue was triturated with diethyl ether to afford 5-chloromethyl-1,2,4-oxadiazol-3-yl)-pyridine, as step-3 product as a off white solid in 96% (16 g) yield. MS: m/z: 197.6 (M+1).
References:
US2009/247478,2009,A1 Location in patent:Page/Page column 46-47
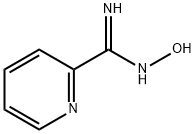
1772-01-6
130 suppliers
$10.00/1g

79-04-9
350 suppliers
$12.00/5g
![2-[5-(Chloromethyl)-1,2,4-oxadiazol-3-yl]pyridine](/CAS/GIF/90002-06-5.gif)
90002-06-5
39 suppliers
$63.00/50mg

749932-24-9
0 suppliers
inquiry
![2-[5-(Chloromethyl)-1,2,4-oxadiazol-3-yl]pyridine](/CAS/GIF/90002-06-5.gif)
90002-06-5
39 suppliers
$63.00/50mg

1772-01-6
130 suppliers
$10.00/1g

79-04-9
350 suppliers
$12.00/5g
![2-[5-(Chloromethyl)-1,2,4-oxadiazol-3-yl]pyridine](/CAS/GIF/90002-06-5.gif)
90002-06-5
39 suppliers
$63.00/50mg

749932-24-9
0 suppliers
inquiry
![2-[5-(Chloromethyl)-1,2,4-oxadiazol-3-yl]pyridine](/CAS/GIF/90002-06-5.gif)
90002-06-5
39 suppliers
$63.00/50mg