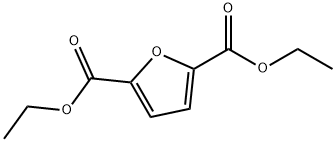
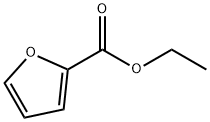
2,5-FURANDICARBOXYLIC ACID DIETHYL ESTER synthesis
- Product Name:2,5-FURANDICARBOXYLIC ACID DIETHYL ESTER
- CAS Number:53662-83-2
- Molecular formula:C10H12O5
- Molecular Weight:212.2
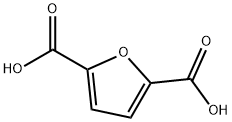
3238-40-2

64-17-5

53662-83-2
To a solution of 2,5-furandicarboxylic acid (1.00 g, 6.41 mmol) in anhydrous ethanol (50 mL) was slowly added 98% aqueous sulfuric acid (0.05 mL), and the reaction mixture was heated and refluxed under nitrogen protection for 18 hours. Upon completion of the reaction, the solution was cooled to room temperature and subsequently concentrated under reduced pressure to remove the solvent. The concentrated residue was separated by filtration and washed sequentially with saturated aqueous sodium bicarbonate solution (3 x 10 mL) and deionized water (3 x 10 mL) to afford finally diethyl furan-2,5-dicarboxylate as a white solid (1.33 g, 98% yield). The structure of the product was confirmed by nuclear magnetic resonance hydrogen spectroscopy (1H NMR, 500 MHz, CDCl3) and carbon spectroscopy (13C NMR, 125 MHz, CDCl3) with the following data: 1H NMR δ 1.38 (t, J = 7.1 Hz, 6H), 4.39 (q, J = 7.1 Hz, 4H), 7.19 (s, 2H); 13C NMR δ 14.2, 61.6, 118.2, 146.9, 158.1.

3238-40-2
473 suppliers
$6.00/1g

64-17-5
825 suppliers
$10.00/50g

53662-83-2
122 suppliers
$6.00/250mg
Yield: 98%
Reaction Conditions:
with sulfuric acid in water at 78; for 67 h;Dean-Stark;
Steps:
12 Synthesis of 2,5-diethyl-2, 5-furandicarboxylate
2,5-furandicarboxylic acid (25.18 g; 160 mmol) was added to ethanol (1,800 mL).Aqueous sulfuric acid (1.32 mL) was added. The mixture was heated at reflux (about78°C) for 67 hours, during which time water was removed from the reaction by the use ofa Dean-Stark apparatus. The reaction progress was monitored using NMR spectroscopy. After the 2,5-diethyl-2,5-furandicarboxylate had been formed in >97% purity by NMR, the reaction mixture was allowed to cool to ambient temperature and was extracted with 2- methyltetrahydrofuran. The combined organic layers were washed with a saturated aqueous brine solution and deionised water, and dried (Mg504). The organics werefiltered and the volatiles were removed in vacuo to afford the title compound (26.77 g; 130 mmol; >98% conversion).
References:
BIOME BIOPLASTICS LIMITED;LAW, Paul;MINES, Paul;CARNELL, Andrew;MCKENNA, Shane WO2016/202858, 2016, A1 Location in patent:Page/Page column 57
6132-37-2
75 suppliers
$27.00/250mg

64-17-5
825 suppliers
$10.00/50g
201230-82-2
1 suppliers
inquiry

53662-83-2
122 suppliers
$6.00/250mg

3238-40-2
473 suppliers
$6.00/1g

64-17-5
825 suppliers
$10.00/50g
32933-01-0
18 suppliers
$106.04/100mgs:

53662-83-2
122 suppliers
$6.00/250mg
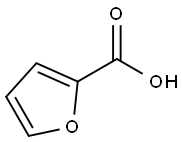
88-14-2
520 suppliers
$5.00/10g

64-17-5
825 suppliers
$10.00/50g

124-38-9
134 suppliers
$175.00/23402

53662-83-2
122 suppliers
$6.00/250mg

64-17-5
825 suppliers
$10.00/50g
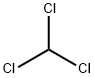
67-66-3
0 suppliers
$33.60/500ml
614-99-3
245 suppliers
$9.00/1g

53662-83-2
122 suppliers
$6.00/250mg